November 15, 2023 feature
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Rapid purification and characterization of circulating small extracellular vesicles on a label-free lab-on-a-chip
All cells secrete nanoscale extracellular vesicles naturally as lipid-bilayer delimited particles. Therefore they are valid biomarkers to identify a variety of diseases.
It is important to efficiently isolate small extracellular vesicles while maintaining the yield and purity to explore their potential in diagnostic, prognostic, and therapeutic applications.
Conventional methods of isolation have shortcomings, which include low purity and yield, lengthy extraction procedures, specialized equipment, and high-costs.
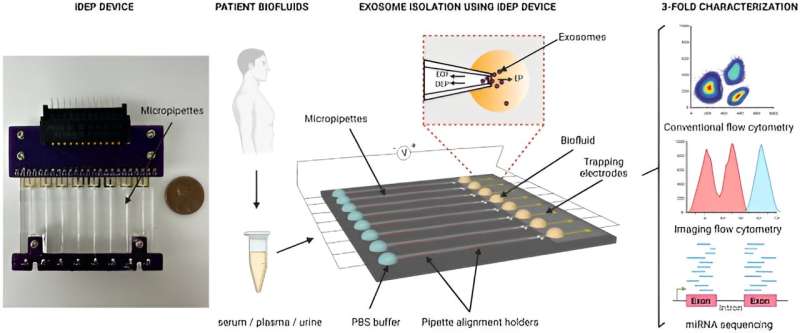
In a study published in Scientific Reports, Manju Sharma and a team of scientists in biomedical engineering at the University of Cincinnati Ohio U.S., developed a new insulator-based dielectrophoretic device to rapidly isolate small extracellular vesicles from biofluids and cell culture media, based on their dielectric properties.
The scientists characterized the small extracellular vesicles isolated from the biofluids of cancer patients using the instrument and conducted a three-fold characterization with conventional flow cytometry, advanced imaging flow cytometry, and microRNA sequencing to obtain a high yield of pure extracellular vesicles. The platform is efficient at rapidly isolating biomarkers and maintaining the biomolecular integrity of the vesicles.
Membrane-encapsulated biological vessels
Biologically, small extracellular vesicles are membrane-encapsulated biological vessels found in biofluids such as blood, urine, saliva, semen, breast milk and cerebrospinal fluid; released by cells into the extracellular space.
Such nanoscale vesicles can horizontally transfer their biomolecular cargo to function as intercellular signaling vectors. Such extracellular vesicles provide a high degree of sensitivity and specificity due to their excellent stability. Their early detection in liquid biopsies can improve the detection of cancers, infections, and neurodegenerative, and metabolic diseases.
The isolation of the vesicles are, however, challenging due to their nanoscale size and physicochemical properties. Isolation methods typically depend on the properties of the extracellular vesicles, and while such devices have promising attributes, their cost of fabrication, sample dilution and susceptibility to clogging are inherent challenges.
In response, Sharma and colleagues developed a class of new insulator-based dielectrophoretic approach with micropillars in microfluidic channels to rapidly engulf nanoparticles based on their size and unique dielectric properties.
Mechanism of action
The device maintained an array of micropipettes that are capable of isolating nanoparticles from small sample volumes by applying a significantly low electric field across the length of the pipettes. The architecture of the pore geometry allowed the isolation of extracellular vesicles from small sample volumes of conditioned cell culture media, and biofluids from healthy donors.
In this work, Sharma and team isolated the biofluids of cancer patients, which included serum, plasma, and urine, followed by multiparametric characterization via flow cytometry and next-generation miRNA sequencing.
-
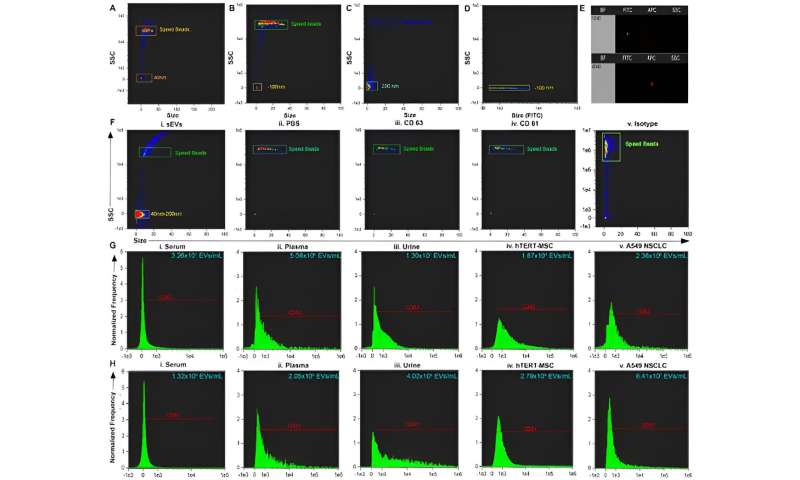
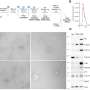
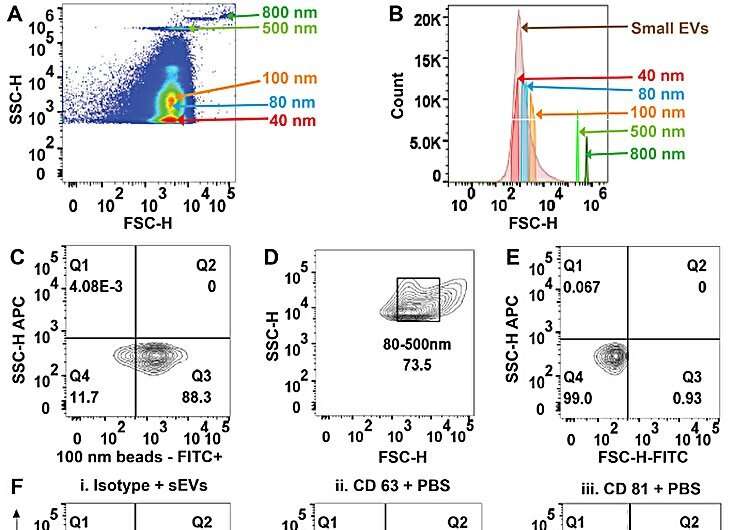
(A–C) Size versus SSC of beads and calibration speed beads. (D) Size versus FITC illustrating the location on side scatter that is positive for fluorescent beads. (E) Representative dot plot of FITC vs. Scatter Intensity (top) and APC vs. Scatter Intensity (bottom). (F) Representative size vs. SSC plot of i. small extracellular vesicles (sEVs) purified from biofluid and negative controls: ii. PBS iii. antibody CD63 iv. antibody CD81 and v. Isotype. (G) Representative histogram of CD63+ sEVs from i. serum, ii. plasma, iii. urine, and positive controls: iv. hTERT-immortalized mesenchymal stem cell (MSC) sEVs, and v. A549 non-small cell lung carcinoma (NSCLC)-derived sEVs. (H) Representative histogram of CD81+ sEVs from i. serum, ii. plasma, iii. urine, and positive controls iv. hTERT-immortalized MSC sEVs, and v. A549 NSCLC sEVs. Credit: Scientific Reports, doi: 10.1038/s41598-023-45409-4 -
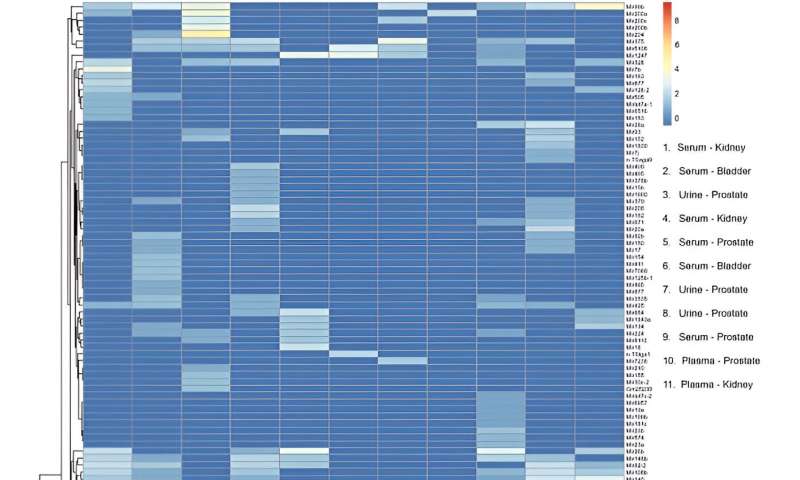
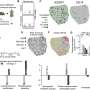
Heatmap of microRNA transcripts isolated from serum, plasma and urine small extracellular vesicles (sEVs) from patients with genitourinary tract cancers. Columns represent individual samples. Legend shows type of biofluid and cancer site for each sample. Rows represent microRNA gene transcripts. Color bar scale represents miRNA enrichment. RStudio Desktop (version 2023.06.2 + 561, accessible at https://posit.co/download/rstudio-desktop/) was used for heatmap generation. Credit: Scientific Reports, doi: 10.1038/s41598-023-45409-4
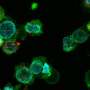
The team purified small extracellular vesicles from serum, plasma, and urine in phosphate buffered saline by using the insulator-based dielectrophoretic approach. Sharma and colleagues used transmission electron microscopy to confirm the presence of the vesicles, and explored multiparametric analysis of purified circulating small extracellular vesicles via flow cytometry.
The team isolated the vesicles and analyzed them, followed by conventional flow cytometry studies. The researchers further showed the capacity and use of the device by characterizing the isolates through ImageStream software.
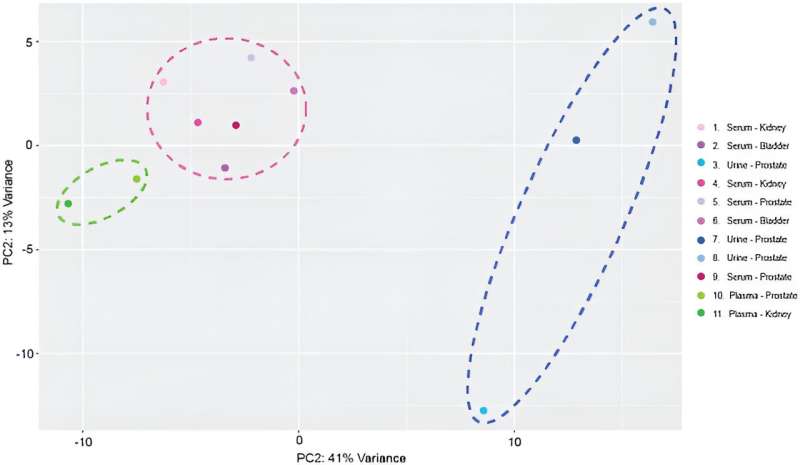
After miRNA sequencing, the team mapped 137 distinct, mature miRNA transcripts to the human genome across samples to include the device in miRNA biomarker analysis workflows. They conducted transcriptomic profiles and performed principal component analysis.
Outlook
In this way, Manju Sharma and colleagues showed the capacity and efficiency of a low-voltage, label-free insulator-based dielectrophoretic device to isolate small extracellular vesicles from serum, plasma, and urine from cancer patients through sub-micron particle detection, and multiparametric characterization by using conventional flow cytometry and advanced flow cytometry methods.
The RNA concentrations of the work were comparable to previous works and affirmed the isolation method to be a viable alternative to those already established in the lab. The analytical methods can be useful as liquid biopsy platforms to isolate small extracellular vesicles, and develop extracellular vesicle-based diagnostic and monitoring platforms.
More information: Manju Sharma et al, Rapid purification and multiparametric characterization of circulating small extracellular vesicles utilizing a label-free lab-on-a-chip device, Scientific Reports (2023). DOI: 10.1038/s41598-023-45409-4
Journal information: Scientific Reports
© 2023 Science X Network