This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Uncovering previously unexplored cellular mechanisms
Single-cell and spatially resolved omics have helped scientists take a closer look at tissue composition, structure, and function. This comprehensive understanding paves the way for them to uncover some intricate and previously unknown disease mechanisms. Three articles in the most recent special issue of the Journal of Pharmaceutical Analysis (JPA) discuss how single-cell and spatially resolved omics help unravel intercellular dynamics, aiding the development of novel therapeutic modalities.
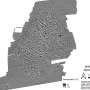
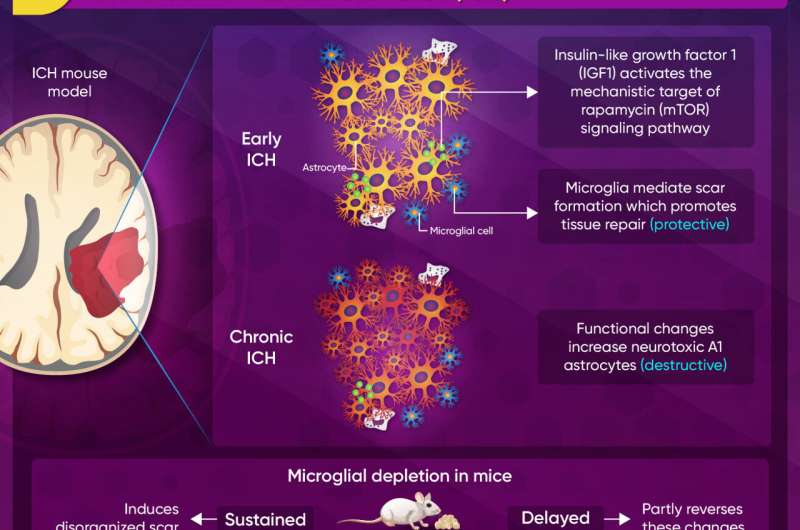
The first study focuses on microglia-astrocyte communication which may improve the therapeutic landscape of intracerebral hemorrhage (ICH). It is based on the notion that after a spinal cord injury or stroke, reactive astrocytes in the brain form a "glial scar," that is lesser known in the context of ICH. To this end, the authors used spatial transcriptomics to investigate how microglial depletion (induced using PLX3397) affects astrocytic scar formation in the early and late stages of ICH.
As documented in the first article, the researchers discovered that during early ICH, microglia-derived insulin-like growth factor 1 (IGF1) regulated protective astrocytic scar formation by activating the mechanistic target of rapamycin (mTOR) signaling pathway, which was further activated by the repopulating microglia.
In chronic ICH, however, the scar had less protective and more neurotoxic effects. Interestingly, this effect could be partially reversed by delayed microglial depletion. The corresponding author Jianmin Zhang says, "Delayed microglial depletion may help with chronic ICH. Combined early-stage IGF1/osteopontin and late-stage PLX3397 treatment could be a promising therapeutic strategy for ICH."
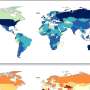
The second study attempted to investigate the testicular toxicity of triptolide, an active component of the traditional Chinese medicine Tripterygium wilfordii at the single cell level. Although triptolide is effective in the treatment of inflammatory diseases, its side effects, particularly testicular toxicity, limit its clinical use. The researchers used triptolide to treat mice, then isolated their testes and prepared single-cell suspensions.
These were used to create a transcriptome map, which revealed that in treated mice, inflammatory response and reactive oxygen species signaling were upregulated, while germ cell and spermatid development were downregulated, indicative of triptolide-induced toxicity. "Our findings provide an invaluable resource for identifying the therapeutic targets of triptolide-induced male reproductive toxicity," explains corresponding author Dr. Chuanbin Yang.
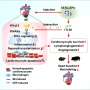
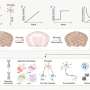
The third study, also published in the same special issue, elaborated on the single-cell and spatial analysis of epicardial cells in mouse heart tissue from embryonic day 9.5 to postnatal day 9.
The team identified that the gene markers Msln, C3, Efemp1, and Upk3b were specific to postnatal (mature) epicardial tissue and were associated with increased extracellular matrix (ECM) signaling. Furthermore, while Wt1, Tbx18, and Upk3b were expressed at all stages, Msln, C3, and Efemp1 were expressed only in mature stages.
"The findings of our research can enhance the current understanding of epicardial cells, contributing to the development of more effective cardiac regenerative therapy strategies. This, in turn, improves the prognosis of patients with myocardial infarction and propels advancements in the field of cardiac repair," comments author Dr. Ling Zhang.
Together, these articles enlighten us with a deeper understanding of cellular mechanisms at the single-cell and spatial levels, offering hope for improved treatment strategies in the future.
More information: Jingwei Zheng et al, Temporal dynamics of microglia-astrocyte interaction in neuroprotective glial scar formation after intracerebral hemorrhage, Journal of Pharmaceutical Analysis (2023). DOI: 10.1016/j.jpha.2023.02.007
Wei Zhang et al, A single-cell landscape of triptolide-associated testicular toxicity in mice, Journal of Pharmaceutical Analysis (2023). DOI: 10.1016/j.jpha.2023.04.006
Jianlin Du et al, Single-cell and spatial heterogeneity landscapes of mature epicardial cells, Journal of Pharmaceutical Analysis (2023). DOI: 10.1016/j.jpha.2023.07.011
Provided by Cactus Communications