This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Researchers describe 'nanoclays,' an innovative addition to tools for chemists
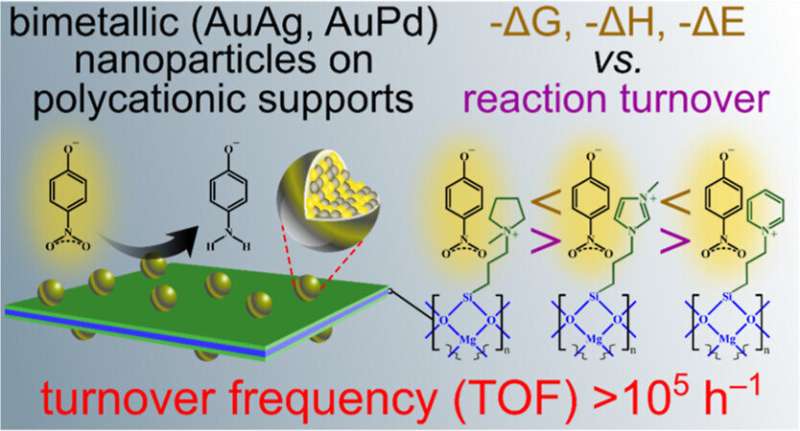
Microscopic materials made of clay, designed by researchers at the University of Missouri, could be key to the future of synthetic materials chemistry. By enabling scientists to produce chemical layers tailor-made to deliver specific tasks based on the goals of the individual researcher, these materials, called nanoclays, can be used in a wide variety of applications, including the medical field or environmental science.
A paper describing this research is published in the journal ACS Applied Engineering Materials.
A fundamental part of the material is its electrically charged surface, said Gary Baker, co-principal investigator on the project and an associate professor in the Department of Chemistry.
"Imagine a koosh ball where the thousands of rubber strands radiating from the ball's core each sport an electrically charged bead on the end," Baker said.
"It's analogous to a magnet—positively charged things will stick to negatively charged things. For instance, positively charged nanoclays could attract a group of harmful fluorinated chemicals known as PFAS, or 'forever chemicals,' which are negatively charged. Or, by making the nanoclay negatively charged, it can stick to things such as heavy metal ions like cadmium, which are positively charged, and help remove them from a contaminated body of water."
In addition to the electrical charge, each nanoclay can be customized with different chemical components, like mixing and matching different parts. This makes them usable in the design of diagnostic sensors for biomedical imaging, or explosive and ordnance detection.
"Essentially, these nanoclays represent chemical building blocks designed with specific functions which are assembled into extremely thin, two-dimensional microscopic sheets—thinner than a strand of human DNA and 100,000 times thinner than a sheet of paper," Baker said.
"We can customize the function and shape of the chemical components presented at the surface of the nanoclay to make whatever we want to build. We've just exposed the tip of the iceberg for what these materials can do."
Two-dimensional materials are highly sought after because they can superficially coat the outside of a bulky object in a thin, conformal layer and introduce completely different surface properties than the object underneath.
"By mixing and matching a few things like different ions or gold nanoparticles, we can quickly design chemistry that's never existed before, and the more we tailor it, the more it opens a wider range of applications," Baker said.
Study co-authors are Nathaniel Larm at the United States Naval Academy, Durgesh Wagle at Florida Gulf Coast University, and Piyuni Ishtaweera and Angira Roy at MU.
More information: Nathaniel E. Larm et al, Surface Programmable Polycationic Nanoclay Supports Yielding 100,000 per Hour Turnover Frequencies for a Nanocatalyzed Canonical Nitroarene Reduction, ACS Applied Engineering Materials (2023). DOI: 10.1021/acsaenm.3c00243
Provided by University of Missouri