June 27, 2023 dialog
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
written by researcher(s)
proofread
Electron tunneling associated with ferritin in vivo in the retina, the cochlea, macrophages and other tissues
Electron tunneling associated with ferritin was proposed as early as 1988, but it is still viewed skeptically despite substantial evidence that it occurs. In our recent paper published in IEEE Transactions on Molecular, Biological and Multi-Scale Communications, my co-authors and I review the evidence of electron tunneling in ferritin, as well as the evidence that such electron tunneling may be used by biological systems that include the retina, the cochlea, macrophages, glial cells, mitochondria and magnetosensory systems.
While these diverse systems fall in different fields of study, we hope that this article will raise awareness of the mechanism of electron tunneling associated with ferritin and encourage further research into that phenomenon in biological systems that incorporate ferritin, particularly where there is no apparent need for the iron storage functions of ferritin in those systems.
A brief history of ferritin and ferritin research
Ferritin is an iron storage protein that self-assembles into a 12-nanometer diameter spherical shell that is 2 nanometers thick, and it can store up to ~4,500 iron atoms in an 8-nanometer diameter core. With an evolutionary history that appears to stretch back more than 1.2 billion years, it might seem rather old, but it should be kept in mind that single-celled organisms are believed to have first evolved ~3.5 billion years ago. As such, it may have taken more than 2 billion years for ferritin to evolve. When the first multicellular organisms evolved ~600 million years ago, members of the ferritin family of proteins were likely present, and they can be found today in almost all plants and animals.
The first suggestion that ferritin might have some quantum mechanical properties was made as early as 1988, 88 years after the discovery of quantum mechanics and eight years after the discovery of quantum dots, semiconductor nanoparticles that behave like artificial atoms and which are similar in size to ferritin. The quantum mechanical properties include magnetic behavior that arises from the way iron forms crystalline structures in the core of the ferritin shell, and electron tunneling.
Subsequent studies discussed in the paper provide substantial evidence that such quantum mechanical properties exist. However, those properties in this billion-year-old biostructure appear to have been mostly considered to be a curiosity or artifact, and not a quantum biological agent. Quantum biology as a study has been viewed with skepticism by biologists and many other scientists (although many of the scientists who discovered quantum mechanics more than 100 years ago believed it could be applicable to biology), but it is a growing field with research being conducted at many of the top universities, such as Caltech, Yale, the University of Chicago and UCLA.
What is electron tunneling?
Quantum mechanics proposes that the physical properties of electrons, protons, neutrons and other things referred to as subatomic "particles" are defined in terms of probability waves. Experimental evidence of the wave-like behavior of these particles has been obtained and is generally accepted. Those waves are described by those experiments as a probability of detecting a physical property of the particle at a location in time and space, which is sometimes referred to as "collapse" of the wave function.
However, nothing changes about the particle when it collapses, other than the behavior of the wave function. When the wave function behaves in accordance with the Schrödinger wave function, it may be called "coherent," and when it interacts with other particles and no longer behaves in accordance with that wave function, it may be called "incoherent."
The spatial wave-like properties of electrons in a vacuum can have a wavelength of around 5 nanometers at room temperature, which is significant for molecular interactions. Electrons can move between molecules when they "touch" each other (recognizing that the wave functions of the atoms and sub-atomic particles in the molecules are what is actually interacting), which may be referred to as adiabatic or classical behavior, but under the right conditions, an electron can "tunnel" between molecules, which means that it can appear to move from one molecule to another molecule in a way that is not permitted by adiabatic or classical behavior. There is nothing mysterious about this, it is just a physical property of electrons, but because the wave function is a probability wave, it can seem mysterious.
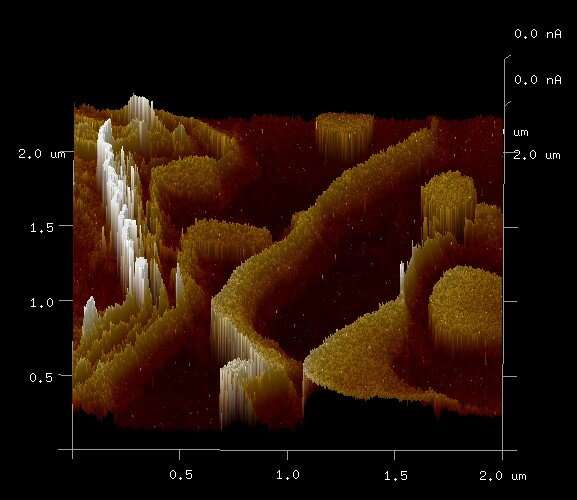
Some of my co-authors have shown that electrons appear to tunnel over distances of up to 12 nm through ferritin in sequential tunneling events, and that the unusual magnetic properties of the core materials of the ferritin might be associated with this unusually long electron tunneling distance. That work has been based on what are referred to as "solid state" experiments, which do not involve living biological systems. Because electron tunneling cannot be directly observed, it has to be inferred from other evidence such as measured currents and voltages. In biological systems, it can be more difficult to obtain evidence of such electron tunneling, but it is not impossible.
Electron tunneling in biological systems containing ferritin
There are several proposed cellular reactions associated with electron tunneling in ferritin. The first is electron storage. In laboratory tests outside of cells, which are sometimes referred to as "in vitro" for the Latin term meaning "in glass," the ability of ferritin in solution with water to store electrons for several hours has been demonstrated. This is unusual, because it would be expected that the iron stored inside the ferritin would be released as soon as an electron is received, but that does not happen quickly. This observation indicates that electrons are not readily conducted through the insulating protein shell by classical conduction, and that instead they move electrochemically or by tunneling.
Evidence also indicates that electrons can tunnel distances of up to 8 nanometers in a single tunneling event through the ferritin in solid state tests, so it is possible that the electrons stored inside the ferritin core can tunnel to molecules outside of the 2-nanometer-thick protein shell, such as free radicals that have energy levels that allow them to receive electrons. These free radicals can take electrons from other molecules and cause cellular damage, and neutralizing free radicals by donating an electron is one of the functions of antioxidants.
Ferritin interacts with antioxidants like ascorbic acid (known more commonly as vitamin C) in a cellular environment in a way that stabilizes the stored iron, and it is also overexpressed in response to free radicals. If ferritin is able to store electrons from antioxidants to make them available to free radicals through electron tunneling, it could improve the efficiency of that neutralization reaction by allowing the electrons to reach free radicals that are farther away and by storing the electrons until they are needed.
If the only function of ferritin is iron storage, that would make no sense in situations where the source of the free radicals, inflammation and ROS is not excess iron, which is often the case. The complexity of the way that cells use iron, known as iron homeostasis, has made it difficult to identify electron tunneling associated with ferritin.
Another proposed quantum biological function for electron tunneling in ferritin is electron transport across cellular distances. In a type of cell called an M2 macrophage, ferritin can form somewhat regularly ordered structures that the macrophages appear to use to provide the ferritin to a cell that the macrophage is aiding. For example, macrophages are associated with increased ferritin levels associated with some cancers and appear to help the cancer cells to neutralize inflammation.
Antioxidants may also help some cancer cells to survive by providing electrons to neutralize free radicals and ROS, but in the absence of antioxidants in those cells, is it possible for electrons to tunnel through the ferritin structures in M2 macrophages into ferritin in other cells? Evidence of that function also exists.
In small angle neutron scattering (SANS) tests performed by Dr. Olga Mykhaylyk on placental tissue that included macrophages, increased neutron scattering was measured that was absent in bulk ferritin extracted from the tissues. Neutron scattering can occur in solids that contain nanoparticles with aligned magnetic moments, and these tests indicate that the ferritin in placental tissue with macrophages has aligned magnetic moments.
SANS tests were also conducted on self-assembled monolayers (SAMs) of ferritin by Prof. Heinz Nakotte that demonstrated neutron scattering, and tests that I conducted with Prof. Cai Shen showed that self-assembled multilayers of ferritin similar to those in M2 macrophages were not only able to conduct electrons over distances as great as 80 microns in vitro but were also able to route those electrons using a physical mechanism known as a Coulomb blockade.
Routing electrons to ferritin where they are need for elimination of free radicals, inflammation and ROS in cells is another proposed quantum biological function, but because electron tunneling cannot be directly observed, further research to investigate that hypothesis is needed.
Conclusion and next steps
This new paper in IEEE Transactions provides more details on how these building blocks of electron tunneling functions could be used in different biological systems that contain ferritin, but it will be up to researchers in the different fields of study for those biological systems to design tests and to investigate whether electron tunneling is occurring.
Many researchers in biology do not understand electron tunneling and are skeptical of quantum biology, so it might take decades before these questions are answered and used to develop treatments for cancer, blindness, deafness and other maladies. Hopefully, this paper will help to raise awareness and foster additional research into whether and how biological systems utilize the proven phenomenon of electron tunneling in ferritin.
This story is part of Science X Dialog, where researchers can report findings from their published research articles. Visit this page for information about ScienceX Dialog and how to participate.
More information: Ismael Diez Perez et al, Electron Tunneling in Ferritin and Associated Biosystems, IEEE Transactions on Molecular, Biological and Multi-Scale Communications (2023). DOI: 10.1109/TMBMC.2023.3275793
Bio: Chris Rourk is a citizen scientist and patent attorney who has been conducting research into quantum biological processes that use electron tunneling associated with ferritin since 2017. He is a former research scientist and received his B.S.E.E. in 1985 and M.Eng. in 1987.