Researchers reveal sorption process of U(VI) on microbial-clay minerals composites surface
Chinese researchers from the Northwest Institute of Eco-Environmental Resources of the Chinese Academy of Sciences (CAS) have systematically studied the enrichment behavior of radioactive element U(VI) on kaolinite- and illite-Aspergillus niger composites, and clarified the regulatory pathways and mechanisms of Aspergillus niger on the sorption behavior of U(VI) on both kaolinite and illite.
This study is of great practical significance to carry out research on the sorption mechanism of uranium on microorganism-clay mineral composites, which helps to reflect the environmental behaviors of uranium accurately.
It is well known that uranium (U) is a kind of radionuclide with chemical toxicity. Once entering the soil system, it will inevitably undergo physical, chemical and biological effects, which is extremely important to the geochemical behaviors of U.
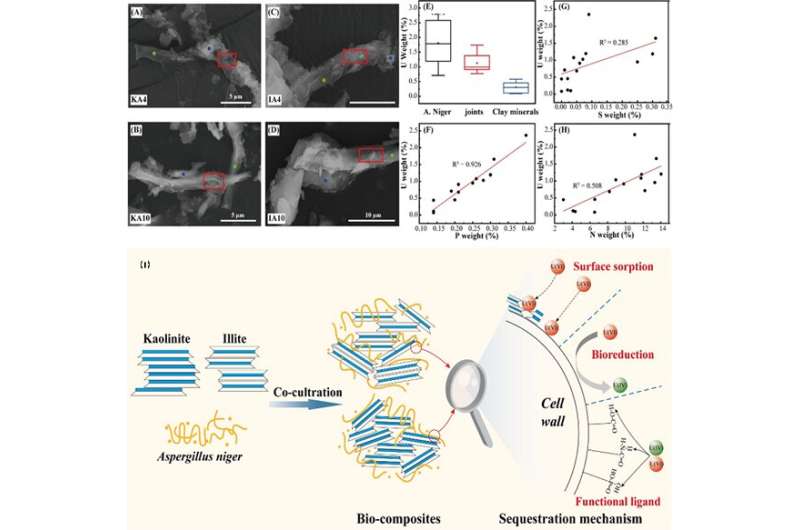
In this study, the researchers found that under different pH conditions, the sorption mechanism of U(VI) on kaolinite and illite is mainly attributed to ion exchange, outer-sphere complexes (OSCs), and inner-sphere complexes (ISCs).
Aspergillus niger could obviously change the charge density, sorption sites and other physical and chemical properties of illite and kaolinite to a certain extent, thereby promoting the sorption of U(VI).
At the same time, the sorption mechanism of U(VI) on the surface of the clay- Aspergillus niger composites could be only attributed to the ISCs. It showed that the sorption process of U(VI) on the bio-composites is significantly different from clay minerals.
The study also showed that U(VI) mainly complexed with the phosphate group, amide group and carboxyl group derived from the biomass of Aspergillus niger in the bio-composites, which further weakened the effect of ion exchange and complexation with Si-OH and Al-OH on illite and kaolinite.
Besides, according to the X-ray photoelectron spectroscopy, U(VI) was partly reduced to non-crystalline U(IV) by Aspergillus niger in the bio-composites.
The findings further clarify the geochemical process of U(VI) in a complicated environmental system, and provid an important theoretical basis for accurately assessing and predicting the environmental behavior of uranium.
This research has been published in Chemosphere in an article entitled "New insights into the sorption of U(VI) on kaolinite and illite in the presence of Aspergillus niger."
More information: Rongyue Geng et al, New insights into the sorption of U(VI) on kaolinite and illite in the presence of Aspergillus niger, Chemosphere (2021). DOI: 10.1016/j.chemosphere.2021.132497
Journal information: Chemosphere
Provided by Chinese Academy of Sciences


















