October 8, 2021 report
An electrolyte design strategy for making divalent metal batteries
A team of researchers working at the University of Maryland has developed an electrolyte design strategy for making divalent metal batteries. In their paper published in the journal Science, the group describes solving problems associated with divalent rechargeable metal batteries and the strategy they developed to overcome them. Pengjian Zuo and Geping Yin with the Harbin Institute of Technology outline issues with the development of divalent metal batteries and describe the work done by the Maryland team in a paper published in the same journal issue.
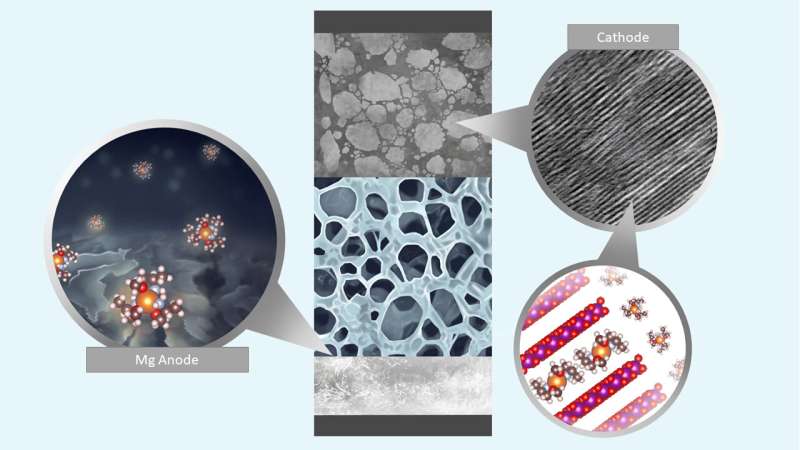
The atoms of divalent metals can combine with dual hydrogen atoms. Some of the most well-known are calcium and magnesium—two metals that are far more abundant and easily accessed than lithium, which is commonly used in batteries. So researchers have been looking for a way to use them in rechargeable batteries. Low working voltages and less than desired cycling performance are obstacles due to the lack of an electrolyte that does not form layers on the anode. There have also been issues with metal migration into the cathode. In this new effort, the researchers have developed a design strategy that overcomes these problems for magnesium.
The strategy involved the use of a versatile electrolyte design in which chelating agents interact with cations, which improved the reversibility of the battery and its charge-transfer kinetics. The researchers noted that magnesium's solvent interface is generally stable compared to lithium. That led them to look for and find a group of methoxyethyl-amine chelants that tend to promote charge transfer without side reactions, as the ligands attach to the atoms in the metal in multiple locations. In testing, the batteries using the chelants, were capable of stable, reversible cycling of both RCB and RMB cells, and they had both high density and high efficiency as well. The researchers suggest that their work provides a design strategy for using divalent metals to make workable, rechargeable batteries.
More information: Singyuk Hou et al, Solvation Sheath Reorganization Enabled Divalent Metal Batteries with Fast Interfacial Charge Transfer Kinetics, Science (2021). DOI: 10.1126/science.abg3954. www.science.org/doi/10.1126/science.abg3954
Pengjian Zuo et al, Chelated electrolytes for divalent metal ions, Science (2021). DOI: 10.1126/science.abi6643. www.science.org/doi/10.1126/science.abi6643
Journal information: Science
© 2021 Science X Network