March 10, 2021 report
Two Frank–Kasper phases in salt crystal structures observed
An international team of researchers has observed two FK phases in the crystal structures of a derivative of pyridine. In their paper published in the journal Nature, the group describes their work with hydrochloride salt of fampridine that crystallized into four different structures. Kathryn Ashe, a writer for Nature has published a News & Views piece in the same journal issue outlining Frank–Kasper (FK) phases and where they are found and the work done by the researchers in this new effort.
As the researchers note, spontaneous self-assembly of simple molecules into those that are more highly structured is very common in the natural world. It occurs in crystals, for example, and proteins and colloids. Because of their nature, such molecules have been used to create certain materials with desired properties. Despite their widespread use, the self-assembly process is still not very well understood. In this new effort, the researchers sought to learn more about the process by working with a particular type of hydrochloride salt (a derivative of pyridine)—one that they discovered crystalizes into four different structures depending on the conditions present during crystallization. Ashe notes that it is rather interesting to see that one organic salt can grow into crystals with such different kinds of structures.
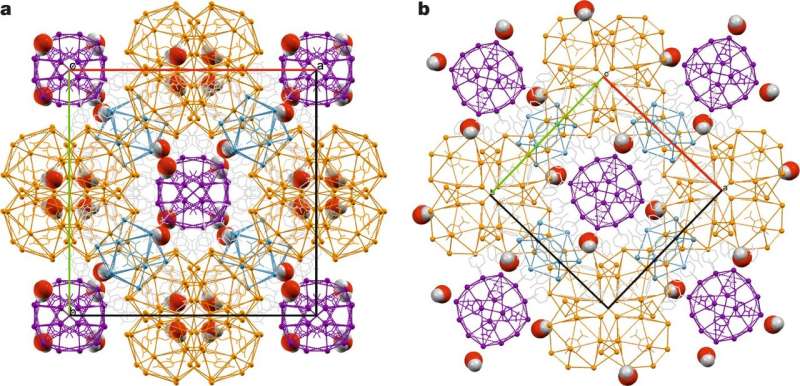
Notably, two of the structures the researchers observed had self-assembling FK phases. FK phases are a family of structures that are closely packed and come about from ordered particles that are spherical in shape. The researchers describe the two they observed as having a dense liquid phase with a degree of complexity that is not generally seen in organic molecules. They were made of pyridinium and chloride ions and had formed in polyhedral clusters. The researchers found that they have spherical aggregates with multiple sizes ranging from 1.5 to 4.6 nanometers. The researchers watched as the structures grew and separated them into three phases. They found that Phase 1 exhibited a degree of complexity that suggested pre-organization prior to crystallization. They suggest that interactions in the systems between solutes and solvents likely had some degree of influence on the final shape of the structures. They note that more work is required to determine if the size of the family of FK phases can be extended.
More information: Riccardo Montis et al. Complex structures arising from the self-assembly of a simple organic salt, Nature (2021). DOI: 10.1038/s41586-021-03194-y
Journal information: Nature
© 2021 Science X Network