August 10, 2020 report
De novo synthesis of a wide range of nucleoside analogs using simple achiral starting materials
A team of researchers from Simon Fraser University and Merck & Co. has developed a de novo synthesis technique for creating a wide range of nucleoside analogs using simple achiral starting materials. In their paper published in the journal Science, the group describes their technique and ways it can be used. Gavin Miller with Keele University has published a Perspective piece in the same journal issue outlining the importance of finding new ways to synthesize nucleosides and the work done by the team in this new effort.
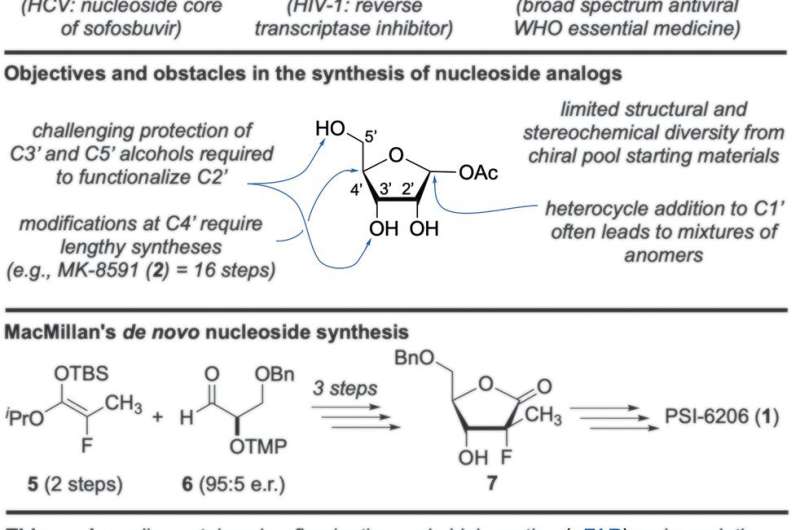
Nucleosides are compounds made of a pyrimidine or purine base linked to a sugar, and as Miller notes, they are the building blocks that make up both RNA and DNA. Because of their importance, scientists have been working for many years to create nucleoside analogs, which could lead to therapies such as preventing cancerous cells from multiplying or virus cells from replicating. Much progress has been made in developing nucleoside analogs. They are currently used to create drugs targeting certain cancers and viruses such as HIV and hepatitis C. But despite progress, more work is required to find new ways of creating them so that they can be used in other applications. In this new effort, the researchers have developed a new de novo synthesis technique to create a wide variety of nucleoside analogs, and it does so using simple achiral (those that are superimposable on their own mirror image) starting materials.
The new technique involved beginning with a heteroaryl aldehyde and a ketone. Next, the researchers used a prolinemediated organocatalytic alpha-fluorination, followed up by an aldol reaction, resulting in the production of a fluorohydrin. That intermediate was then converted to a nucleoside analog scaffolding using an annulative fluoride displacement strategy, forming a sugar ring product (a nucleoside analog building block). The researchers note that the method results in analogs with the proper spatial orientation of the atoms in the ring and allows for high production yields and high purity.
The researchers tested their technique by using it to create a wide variety of derivatives, including nucleosides that had different nucleobases.
More information: A short de novo synthesis of nucleoside analogs, Science 07 Aug 2020: Vol. 369, Issue 6504, pp. 725-730 DOI: 10.1126/science.abb3231 , science.sciencemag.org/content/369/6504/725
Journal information: Science
© 2020 Science X Network