Orderly arranged bead-chain ternary nanocomposites for supercapacitors
In a paper published in Nano, a group of researchers from Jiangsu University of Technology, China have developed novel Cu2O-Mn3O4-NiO ternary nanocomposites by electrostatic spinning technology, which improved the performance of supercapacitor electrode materials.
Supercapacitors feature high power density, long cycle life and present increasing significance as advanced energy storage devices. Nanomaterials and their composites are recognized as optimal candidates for energy materials because of their ease in charge conduction mechanisms, reduced dimensions and the effect of surface properties on their behavior provide better interfaces and chemical reaction rates.
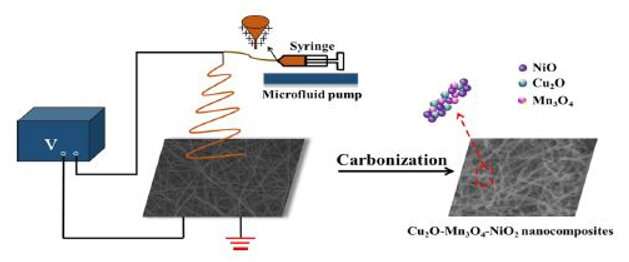
However, the preparation of electrode materials is a key point affecting the performance of supercapacitors. When compared with other methods for fabricating nanofibers, electrospinning has attracted more and more attention because of its single step and cost-effectiveness. Electrospinning metal oxide fibers is a promising method for generating composite nanofibers with a high specific surface area, high crystallinity, and an increased number of active sites. The resultant nanofibers are ideal for energy storage applications because the nanofibrous surface morphology provides a path for electron transport, which improves the energy storage capacity of the metal oxide.
In this work, the obtained nanocomposites (Cu2O-Mn3O4-NiO) are an ordered arrangement of metal oxide particles (10 nm), with the shape like a bead-chain. The acquired Cu2O-Mn3O4-NiO ternary nanocomposites were used as electrode materials to manufacture a supercapacitor. Electrochemical tests showed that the synthesis of nanocomposites-made electrode materials had good electrochemical performance in 6 mol/L KOH electrolyte. The results showed that at a scan rate of 5 mV/s, the specific capacitance of Cu2O-Mn3O4-NiO had a larger specific capacitance of 1306 F/g than NiO, Cu2O-NiO and Mn3O4-NiO. This ternary nanocomposites improved the electrochemical performance of electrode materials and can be used for efficient supercapacitors.
The successfully synthesized Cu2O-Mn3O4-NiO nanocomposites by electrospinning is adaptable for large and industrial scale production. The structural characterization and composition analysis explained the excellent behavior of Cu2O-Mn3O4-NiO. Due to the chemical reactions and hence strong interaction between the functional groups and electrolyte ions, Cu2O-Mn3O4-NiO nanocomposites exhibited outstanding electrochemical performance in terms of high specific capacitance and capacitance retention.
More information: Lei Su et al, Orderly Arranged Bead-Chain Cu2O-Mn3O4-NiO Ternary Nanocomposites with High Specific Capacitance for Supercapacitors, Nano (2020). DOI: 10.1142/S1793292020500824
Provided by World Scientific Publishing