May 28, 2020 report
A better way to split water molecules to produce hydrogen using sunlight
A team of researchers affiliated with several institutions in Japan has developed a better way to split water molecules to produce hydrogen using sunlight. In their paper published in the journal Nature, the group describes their technique and how well it worked. Simone Pokrant with Inscripta, Inc. has published a News and Views piece outlining the problems that scientists have faced in trying to use sunlight for electrolysis and also details the work by the team in the same journal issue.
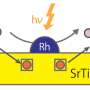
As the planet continues to grow warmer due to the continuation of greenhouse gas emissions, scientists seek alternatives to burning gasoline in cars—a major contributor to global warming. One major area of research has involved replacing gasoline in cars with hydrogen—when it combusts, it does not produce any greenhouse gases. But such efforts have been stymied by efficiency and economic issues. In this new effort, the researchers have taken a new look at using strontium titanate, an oxide of strontium and titanium. Scientists have known since the late 1970s that it can be used to split water molecules photocatalytically, but have been unable to find an economical way to use it. The researchers in Japan have found ways around several of the hurdles to its general use.
The researchers applied several new techniques to the use of strontium titanate as a photocatalyst. The first involved charge recombination suppression by improving crystallinity and lessening the number of chemical defects in the crystal lattice. The second technique involved additional suppression of charge recombination by selectively depositing co-catalysts on the crystal facets. A third technique involved preventing undesirable side-reactions by covering the rhodium co-catalyst in a protective casing made of a chromium compound.
The combination of improvements to the technique led to a higher external quantum efficiency score (the fraction of the photons striking the reaction that the photocatalyst can use to split water molecules)—they achieved 96% when using their technique with the photocatalyst when testing with irradiated light. More work is required before their technique can be translated to real-world conditions, but the researchers suggest their work shows that such an approach is viable.
More information: Tsuyoshi Takata et al. Photocatalytic water splitting with a quantum efficiency of almost unity, Nature (2020). DOI: 10.1038/s41586-020-2278-9
Journal information: Nature
© 2020 Science X Network