October 3, 2019 report
A safer way to make azides for use in click chemistry
A team of researchers at the Chinese Academy of Sciences has found a safer way to synthesize azides for use in click chemistry reactions. In their paper published in the journal Nature, the group describes how they discovered a safer way to transform primary amines into azides. In the same journal issue, Joseph Topczewski and En-Chih Liu with the University of Minnesota have published a News & Views piece outlining the work by the team in China.
Click chemistry combines two reactive compounds that assemble in ways that reliably produce results with no byproducts. The most popular click reaction is called CuAAC and it is used in a wide variety of applications. But synthesizing an azide for use in the process is problematic—it takes a very long time, and produces toxic emissions. Also, the reagents pose an explosion risk when stored. In this new effort, the researchers report that they have found a new way to synthesize azides that eliminates both problems.
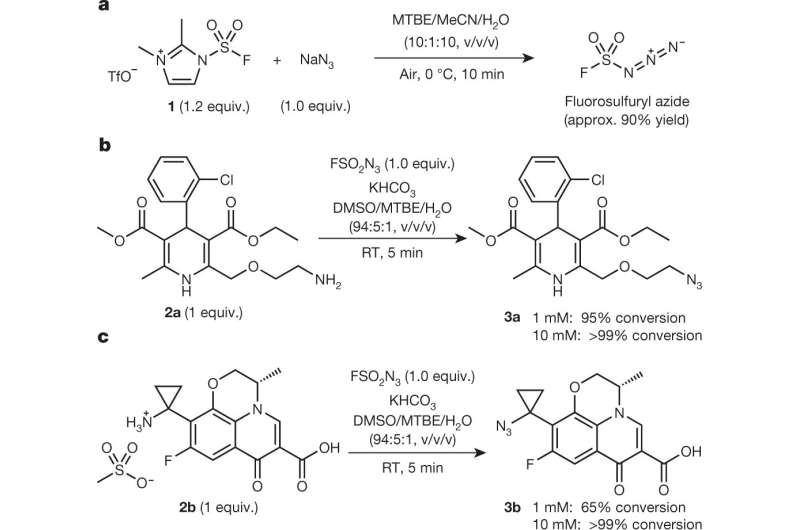
The researchers report that they were actually working on something else when they discovered that fluorosulfuryl azide (FSO2N3) could convert primary amines into azides—and instead of it taking hours, it took just a few minutes. Further testing showed that FSO2N3 would react with almost any primary azine, and that it almost always resulted in a 100 percent yield. The researchers note that there is no need to purify FSO2N3 before use, making it an inexpensive option. They further note that there is no need to store it because it can be produced when needed—by mixing imidazolium fluorosulfuryl triflate salt with a sodium azide. They also note that the initiating salt is not toxic—at least to test rodents that were exposed to it.
Topczewski and Liu suggest the work by the team in China could open the door to very efficient and generalized two-step methods for converting primary amines into triazoles. They further note that the work also represents another step toward the ultimate goal of click chemistry—developing just a few reactions that can be used to create precursors for a wide number of applications.
More information: Genyi Meng et al. Modular click chemistry libraries for functional screens using a diazotizing reagent, Nature (2019). DOI: 10.1038/s41586-019-1589-1
Journal information: Nature
© 2019 Science X Network