April 17, 2019 feature
Direct imaging of active orbitals in quantum materials
In quantum materials based on transition metals, rare-earth and actinide elements, electronic states are characterized by electrons in orbitals d and f, combined with the solid's strong band formation. Until now, to estimate the specific orbitals that contribute to the ground state of these materials and determine their physical properties, researchers have primarily relied on theoretical calculations and spectroscopy methods.
In a recent study published in Nature Physics, a team of researchers at Max Planck Institute Dresden, Heidelberg University, University of Cologne, and DESY- Hamburg attempted to image a material's active orbitals directly in real space, without any modeling. The imaging technique they devised is based on s-core level and non-resonant inelastic X-ray scattering.
"We are interested in how materials attain their properties," Hao Tjeng, one of the researchers who carried out the study, told Phys.org. "We want to know how these can be explained on the basis of the behavior of the electrons in the materials. We are mostly interested in transition metal (3d, 4d, 5d) and rare-earth-based (4f) materials, since they offer a wealth of fascinating and tunable properties, important for fundamental science and for numerous other applications."
When they first started working on their study, Tjeng and his colleagues knew that the quantum mechanical equations that they would need to solve were unsolvable, as the relevant calculations would take an infinite amount of time. They thus realized that it would be far more practical and useful to image the orbitals in practical experiments.
"Usually, in order to determine what type of quantum mechanical states are realized in a material, one carries out spectroscopic measurements," Tjeng explained. "These have their merits, but also their limitations: one still need to do calculations to extract the information, and quite often the results are not accurate or reliable. We were thus looking for a new method that can provide a direct image of the quantum mechanical state straight for the experiment. Maurits Haverkort and I realized that inelastic x-ray scattering could provide such an opportunity."
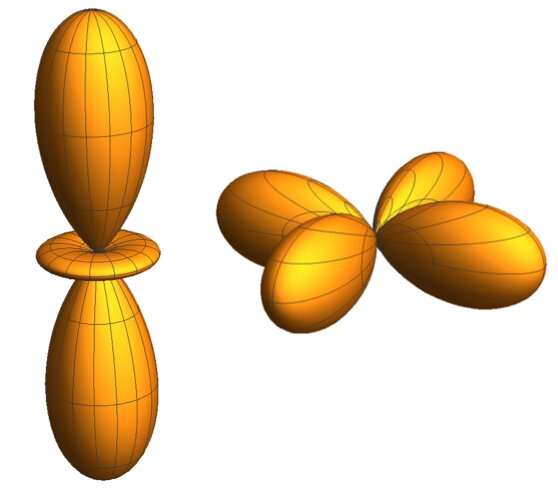
Using X-rays and large momentum transfers, the researchers were able to observe atomic transitions in the sample that would otherwise be forbidden in standard experiments, such as x-ray or optical absorption spectroscopy. Haverkort and Tjeng realized that by making a transition from a spherical atomic state (e.g. 3s) they could attain the shape of a 3d orbital with respect to the photon momentum transfer.
"Initially, all of this was theory," Tjeng said. "We then set out to do the experiment, investing and upgrading an existing instrument at the PETRA-III synchrotron facility, in order to have sufficient signal, considering that this is a very photon hungry experiment. After some efforts, we were indeed able to observe the signal and the results that we had envisioned."
In their experiment, Tjeng ad his colleagues used synchrotron radiation as an 'undulator' beamline, to deliver monochromatic x-rays with high intensities. They directed the x-ray beam at a sample, specifically a single crystal; then they detected and analyzed the scattered x-rays.
"By looking at the intensity of a particular atomic process (in our case 'the 3s-to-3d excitation') as a function of the orientation of the sample with respect to the transferred photon-momentum and by displaying these intensities on a polar plot, we obtained a direct image of the 3d orbital.," Tjeng said.
In their study, Tjeng and his colleagues were able to demonstrate the effectiveness, both in terms of power and accuracy, of the imaging technique proposed by them. They successfully applied their method on a textbook example, the x2y2/3z2-r2 orbital of the Ni2+ ion in a NiO single crystal.
"By being able to directly image the orbitals that are active in a material, we will have a better and more precise insight in the behavior of the electrons that are responsible for the properties of the material," Tjeng said. This is especially important for the design of new materials with new or optimized properties, which is highly desired by both the physics and chemistry research communities."
Tjeng and his colleagues have presented a tangible and efficient alternative to current methods for studying orbitals in quantum materials, which could ultimately enhance research in both physics and chemistry. In their future work, they plan to use their technique to study other complex materials. In addition, they would like to improve the apparatus and instruments employed by their method, so that it can become a standard source of measurement, such as single crystal x-ray or neutron diffraction measurement.
More information: Hasan Yavaş et al. Direct imaging of orbitals in quantum materials, Nature Physics (2019). DOI: 10.1038/s41567-019-0471-2
Journal information: Nature Physics
© 2019 Science X Network



















