'Frozen' copper behaves as noble metal in catalysis: study
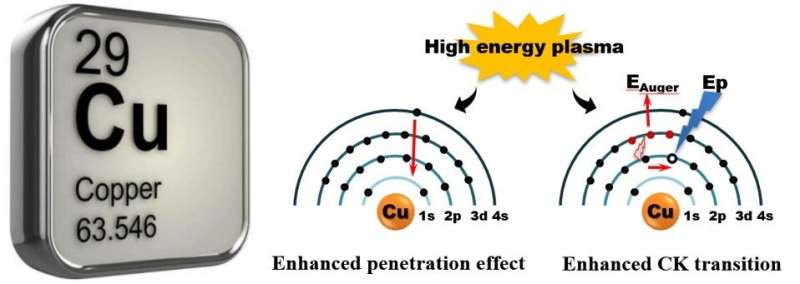
As a non-noble metal, copper oxidizes more easily to a positive valence (Cu+ or Cu2+) than same-family elements Au or Ag. In general, this chemical property is mainly determined by electron structure. Can we change the chemical properties of an element by regulating its electron structure? Can Cu act as a noble metal in catalytic reactions?
A team led by Dr. Sun Jian of the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) gives a positive answer. The team's recently published paper in Science Advances shows that the electron structure of Cu can be changed, assisted by high energy plasma, making Cu exhibit significantly different catalytic behaviors than normal Cu in selective hydrogenation reactions.
The dimethyl oxalate (DMO) hydrogenation reaction, a typical multistep catalytic reaction producing methyl glycolate (MG), ethylene glycol or ethanol, was selected as a probe reaction for copper. In this reaction, the common product over supported Cu/SiO2 catalysts is one of the latter two owing to the inevitable co-existence of Cu+ and Cu0 for deep hydrogenation.
The sputtered (SP) Cu, which is bombarded by high energy argon plasma, can be "frozen" at zero valence when exposed to oxidation or reaction atmosphere at a very wide range of temperature, presenting noble-metal-like behaviors.
In DMO hydrogenation, a high selectivity (87%) towards the preliminary hydrogenation product, MG, a high-value chemical, was observed. The molecule level free energy surface in various reaction pathways by DFT calculation also verifies that "frozen" Cu0 is crucial for preliminary hydrogenation.

More information: "Freezing copper as a noble metal–like catalyst for preliminary hydrogenation" Science Advances (2018). DOI: 10.1126/sciadv.aau3275 , advances.sciencemag.org/content/4/12/eaau3275
Journal information: Science Advances
Provided by Chinese Academy of Sciences





















