Making vanilla flavoring with less pollution
In small amounts, vanilla flavoring enhances the taste of our baked goods, desserts and ice cream. But making it synthetically, which is the most common route to keeping the ingredient affordable these days, creates a stream of wastewater that requires treatment before it can be released into surface waters. Now researchers report in ACS' journal Industrial & Engineering Chemistry Research a new "greener" way to make vanillin, the primary flavor compound in vanilla.
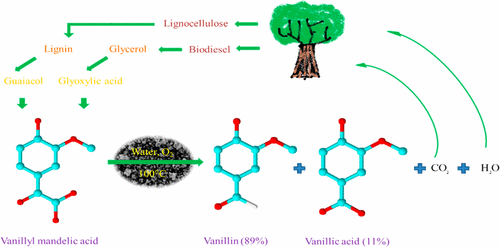
Although consumers have been demanding more "natural" foods in recent years, less than 1 percent of vanilla flavor produced globally comes from its original natural source, the vanilla orchid. The rest is synthesized from a petroleum-derived precursor called guaiacol, tree lignin and other substances such as cow feces. But the catalysts currently used in the manufacturing of vanillin are polluting and can only be used one time. So Ganapati D. Yadav and Shivaji L. Bhanawase sought an improved method to make the popular flavor compound.
The researchers created a catalyst by encapsulating copper-aluminum hydrotalcite in silica. Testing showed that it efficiently spurred the separation of vanillin from other compounds. The catalyst worked in water under ambient air pressure, and eliminated the need for a polluting step involving hydrochloric acid that current techniques require. The catalyst could also be recovered and re-used. The researchers say that their process could be economically scaled up for a more environmentally friendly approach to making commercial vanillin.
More information: Shivaji L. Bhanawase et al. Novel Silica-Encapsulated Cu–Al Hydrotalcite Catalyst: Oxidative Decarboxylation of Vanillyl Mandelic Acid to Vanillin in Water at Atmospheric Pressure, Industrial & Engineering Chemistry Research (2017). DOI: 10.1021/acs.iecr.6b04982
Abstract
Vanillin is a versatile chemical, having characteristics such as antimicrobial, antioxidant, anticarcinogenic, antimutagenic, hypolipidemic, and antisickling activities and is an intermediate for synthesis of fine chemicals such as vanilla flavoring. Vanilla is the second most expensive spice; it is extracted from vanilla beans and is in short supply. Therefore, to meet demand of vanilla flavor, the synthesis of vanillin via chemical routes is necessary. In this regard, a novel silica-encapsulated copper aluminum hydrotalcite (SECuAlHT) was synthesized. Different techniques such as XRD, FTIR, N2 adsorption–desorption, TGA-DSC, NH3 TPD, CO2 TPD, H2 chemisorption, TEM, and SEM-EDXS were used to characterize SECuAlHT. Its catalytic activity for oxidative decarboxylation of vanillyl mandelic acid (VMA) was investigated. Vanillin was obtained from VMA by oxidative decarboxylation using SECuAlHT in water as a solvent and atmospheric air under base-free conditions. The presence of basic sites in the catalyst was confirmed with CO2-TPD. Effect of different reaction conditions on catalyst activity and selectivity for oxidative decarboxylation of VMA was investigated. Vanillin was efficiently obtained with 89% selectivity at 81% VMA conversion over the SECuAlHT catalyst at 100 °C in 8 h. The catalyst was active, selective, stable, and reusable. Catalytic cycle based on the Mars van Krevelin mechanism was proposed and the reaction kinetics studied. It is a zero-order reaction. The apparent energy of activation was 8.7 kcal/mol. Thus, vanillin was efficiently synthesized from VMA via a green catalytic route in water as a solvent.
Journal information: Industrial & Engineering Chemistry Research
Provided by American Chemical Society