Forging a brand-new chemical bond using the pressure of the Mars core
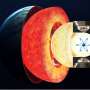
When it comes to making chemical bonds, some elements go together like peanut butter and jelly; but for others, it's more like oil and water. Scientists can combat this elemental antipathy using extreme pressures. And now in ACS Central Science, researchers report that they have used pressure equivalent to that within the core of Mars to forge the first-ever iron-bismuth bond, which could help them make brand-new magnetic and superconducting materials.
For most reactions, the first step is to mix the "ingredients" evenly, which is unbelievably difficult to do with iron and bismuth. Even at nearly 3,000 degrees Fahrenheit—a temperature hot enough to melt both metals—only 0.16 percent of the bismuth will dissolve into the molten iron.
Danna Freedman and colleagues proposed using very high pressure to make the two elements more amenable to bonding. At pressures around 30 GPa, the researchers observed evidence of a new substance: FeBi2.
They found they could lower the pressure to 3GPa and still maintain the material, although back at earth's atmospheric pressure (nearly 30,000 times lower) the compound returns to its constituent parts.
Freedman notes that her group is currently working on approaches to scale up the synthesis to allow them to further investigate whether this unique compound is superconductive and magnetic, as they predict it could be and find ways to make it stable.
More information: ACS Central Science, pubs.acs.org/doi/full/10.1021/acscentsci.6b00287
Journal information: ACS Central Science
Provided by American Chemical Society