Slow-binding inhibition of cholinesterases
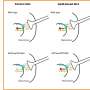
Reversible inhibition of an enzyme, an activity in which the inhibiting molecular entity (often a small chemical called ligand or inhibitor) associates and dissociates from the protein's binding site, is a very fast process. During enzyme-substrate interaction, enzyme-inhibitor equilibrium is established within microseconds. Thus, classical reversible inhibitors are characterized by rapid on/off rates. However, a number of enzymes do not respond instantly to reversible inhibitors. In such cases, there is a slow onset of inhibition. This type of reversible inhibition is called slow-binding inhibition (SBI) and is characterized by slow establishment of enzyme-inhibitor equilibrium.
Because SBIs slowly bind and slowly dissociate from their target, several kinetic parameters are useful to describe toxico/pharmacodynamic processes, enabling an understanding of the endogenous mechanisms of protection against SBI toxicants, and facilitating the discovery of new pharmacological countermeasures. These parameters also account for the efficacy of SBIs as drugs.
Researchers from Laboratory of Neuropharmacology, Kazan Federal University, and Laboratory of Computer Modeling of Biomolecular Systems and Nanomaterials, Emanuel Institute of Biochemistry of RAS conducted a study dedicated to cholinesterases (ChEs), which display slow onset of inhibition with certain inhibitor. The study highlights the pharmaco-toxicological importance of SBI of these enzymes.
The fully integrated study included synthesis of the molecules, enzyme and inhibitory kinetics, crystallography, molecular modeling, pharmacokinetics and pharmacodynamics.
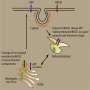
Thus, one of the SBI's of this nature, acetylcholinesterase, has an important physiological function in terminating the action of the neurotransmitter acetylcholine in the central cholinergic system, ganglia and at neuromuscular junctions. AChE has also non-cholinergic functions in cell development and embryogenesis, and is involved in pathogenesis of Alzheimer's disease in promoting formation of beta-amyloid fibrils. Inhibitors of AChE have been used for the palliative treatment of Alzheimer's disease, glaucoma and myasthenia. Irreversible inhibition of AChE by organophosphorus compounds (mostly pesticides and chemical warfare nerve agents) and carbamates causes a major cholinergic syndrome, responsible for the acute toxicity of these compounds.
The related enzyme, butyrylcholinesterase (BChE) has no known physiological function, though it has recently been found to hydrolyze ghrelin, a neuropeptide also called the "hunger hormone." Otherwise, BChE is of toxicological and pharmacological importance. It acts as an endogenous bioscavenger against numerous esters used as drugs, pesticides, or banned chemical warfare agents.
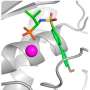
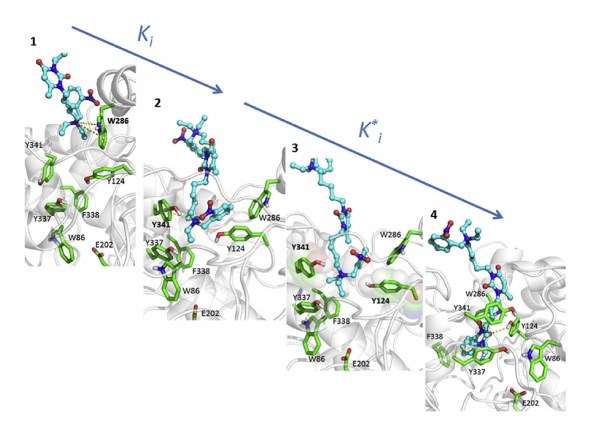
Certain potent reversible inhibitors bind slowly to the active center of both ChEs. Slow onset inhibition determines kinetic complexities in terms of possible mechanisms of protection against external toxicants, pharmacological uses of slow-binding inhibitors and design of new drugs with long residence time on targets and short residence time in the bloodstream.
To analyze and interpret the data, the researchers used new concepts in pharmacology: residence time, rebinding and micro-pharmacodynamics
SBI of ChEs results either from simple slow interaction, induced-fit, or slow conformational selection. In some cases, slow equilibrium is followed by an irreversible chemical step. This later was observed in the interaction of ChEs with certain irreversible inhibitors.
Because slow-binding inhibitors present pharmacological advantages over classical reversible inhibitors (e.g. high selectivity, long target-residence times and rebinding to target in micro sub-organ compartments (e.g. neural synapses and neuromuscular junctions), resulting in prolonged efficacy with minimal unwanted side effects that could lead to a decrease in the number of pills consumed and space out the time between doses taken), slow-binding inhibitors of ChEs, in particular methyl-uracil derivatives that have been synthesized in Kazan, are promising new drugs for treatment of Alzheimer disease, myasthenia, and neuroprotection. SBI is also of toxicological importance; it may play a role in mechanisms of resistance and protection against poisoning by irreversible agent.
To further understand the toxic and/or therapeutic effects of SBI, toxicology/pharmacology and neurophysiology the research group has been conducting experiments in vivo on model animals and isolated cholinergic cell systems and muscles in collaboration with scientists of A.E. Arbuzov Institute of Organic and Physical Chemistry Kazan Scientific Centre of the Russian Academy of Sciences.
More information: Patrick Masson et al. Slow-binding inhibition of cholinesterases, pharmacological and toxicological relevance, Archives of Biochemistry and Biophysics (2016). DOI: 10.1016/j.abb.2016.02.010
Provided by Kazan Federal University