Environmentally friendly polymer coatings inhibit the surface buildup of bacterial and marine organisms

An eco-friendly strategy has been developed by A*STAR researchers to stop the collection of bacteria and marine organisms on objects immersed in seawater. Working under the Innovative Marine Antifouling Solutions program, the scientists have created a safe, polymer-based, coating.
Marine fouling badly damages ships, seawater filtration systems, and harbor installations, and leads to expensive and time-consuming repairs. Fouling also corrodes ship hulls which increases their fuel consumption. It has proven destructive for high-performance devices specific to the maritime industry, such as underwater communication equipment and buoy sensors.
Traditional measures against marine fouling rely on coatings that release substances known as biocides, which deter or kill these microorganisms. But, these compounds also harm the marine habitat, especially in shallow bays and harbors, leaving an extensive ecological footprint.
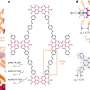
In their search for alternative coatings to biocides, Anbanandam Parthiban, and coworkers from the A*STAR Institute of Chemical Engineering Sciences and Institute of Materials Research and Engineering have discovered so-called poly(methyl oxazoline) (PMOx) polymers that prevent microorganisms from sticking to surfaces and, where there is contact, facilitate their detachment.
According to Parthiban, low-adhesive polymers that form hydration layers on coated surfaces have emerged as potential antifouling agents. "Poly(methyl oxazoline) is the third generation of hydrophilic polymers under focus," he adds. Parthiban described a peptide-like chemical backbone, which shows greater resistance to oxidation than its widely-studied predecessor polyethylene glycol. This makes it attractive for long-term performance—a major challenge in the design of antifouling agents.
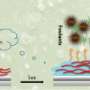
Parthiban explains that PMOx is typically anchored on surfaces through electrostatic interactions, which can be nullified by charge screening in high-ionic-strength solutions, such as seawater. To preempt this issue, the researchers covalently attached the polymer chains to surfaces by curing precursors functionalized with reactive end groups using ultraviolet light.
After an initial reduction in thickness, the coatings remained intact when immersed in a seawater proxy for two months. They effectively reduced the settlement of barnacle larvae and algae regardless of polymer mass and surface charge.
The coatings may also have biomedical applications, as they reduced the attachment of bacteria Staphylococcus aureus and Escherichia coli. "Bacterial adhesion showed a strong response to surface charge," adds Parthiban.
The researchers are talking to potential industrial partners about the possible implementation of these new coatings in high-value applications. Also, in a continued effort to come up with biocide-free technologies, they are creating coating materials that satisfy the diversity of organisms in various water bodies.
More information: Tao He et al. Efficient and robust coatings using poly(2-methyl-2-oxazoline) and its copolymers for marine and bacterial fouling prevention, Journal of Polymer Science Part A: Polymer Chemistry (2016). DOI: 10.1002/pola.27912