Proofreading molecules tug on RNA to ensure protein production accuracy
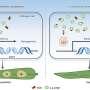
If you imagine a cell as a house, protein production can be a fairly relatable engineering feat. A master blueprint (DNA) holds all the information about what goes where. If you just want to build a door (protein), you only need a copy of that specific portion of the blueprint (messenger RNA, or mRNA). In cells, however, raw mRNA copies contain extra material that is not relevant for the final protein. To remove these superfluous chunks, cells use a process known as splicing, in which raw mRNA is cut up and stitched back together in alternative ways to create the definitive blueprint for a protein.
Splicing is a critical biological mechanism—at least 15 percent of human diseases, including some cancers and neurodegenerative diseases, involve splicing errors. Alternative splicing also significantly increases the number of proteins a single gene can code for, and is thought to explain why, for example, humans are more complex than worms even though we share roughly similar numbers of genes.
Now, University of Chicago scientists have discovered how two enzymes play a critical role in ensuring quality control during splicing. In a study published in Cell on Feb. 25, they found these molecules most likely apply physical tension to RNA to keep wrong sites from being cut. This action not only prevents splicing errors, it also enables the selection of alternative splicing sites.
"These proofreading enzymes effectively pull and yank on RNA, and through that action at a distance, can extract suboptimal sites from the splicing machinery," said senior study author Jonathan Staley, PhD, professor of molecular genetics and cell biology at the University of Chicago. "This suggests a new mechanism for regulating splice site choice, and shifts the perspective on the realm of possible activities for this class of protein. They can move RNA, not just move along it like we previously thought."
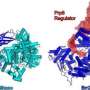
The double-stranded structure of DNA is somewhat analogous to a zipper, with the teeth representing letters of genetic code. For that code to be accessed and read, a class of enzyme known as helicases functions like the zipper slider to separate the two strands. Helicases are also required to "unzip" RNA, which, despite being single-stranded, can also form a double helix. Staley and his team have previously shown two RNA-specific helicases, Prp16 and Prp22, play a role in proofreading the two chemical reactions of splicing. But the mechanism by which they acted was unknown.
Based on previous studies on how Prp16 and Prp22 behave, they guessed that the helicases proofread by moving along the raw mRNA strand. If either encountered the spliceosome—the molecular machine responsible for cutting and re-assembling mRNA during splicing—at the wrong location, the helicases would knock the spliceosome off the RNA strand, preventing an incorrect cut.
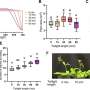
While experiments supported helicase movement, they ruled out the possibility that the helicases were knocking the spliceosome off the RNA. The team used a technique in which they swapped out a short segment of a strand of RNA with DNA, which acted as a barrier to the movement of Prp16 and Prp22. When the segment was positioned near the spliceosome, the helicases were still able to prevent incorrect splicing. But if the segments were positioned further away, incorrect splicing occurred.
Staley and his team propose that the most likely explanation for the ability of the helicases to proofread is physical tension placed on the RNA strand. Instead of directly knocking the spliceosome off an incorrect splice site, the helicases were pulling the site away from the spliceosome.
"Our tests did not support the idea that the helicases were plowing through and dislodging the spliceosome, suggesting that they do not need to go directly to their target to act on it," Staley said. "This led us to propose an alternative model that, in a way... reminds me of special relativity, and requires us to shift our frame of reference."
The researchers also found Prp16 and Prp22 played an important role in allowing the spliceosome to search for alternative splice sites. For example, they designed raw mRNA in a way that allowed the spliceosome to position for an initial cut but then stall due to the inadequacy of the site. Despite this, however, they found that the spliceosome carried out every step of its task—not at the site on the RNA they had originally disabled, but at sites no one had observed before. When Prp16 was completely removed from the spliceosome, this alternative splicing would not occur. If it was added back in, alternative splicing resumed. Prp22 showed a similar behavior.
"We found that these helicases prevent splicing at one site, but enable splicing at another," Staley said. "This is the first evidence that they act like alternative splicing factors and play a role in the process that allows one gene to give rise to multiple protein products."
While still the subject of ongoing research, the researchers believe that the spliceosome is strongly attracted to optimal splice sites and weakly to incorrect ones. When a helicase pulls on the RNA strand, suboptimal sites are easily dislodged, while optimal ones remain stable long enough for splicing to occur.
They are now working to verify this model, as well as looking at ways of manipulating the process. RNA helicases are evolutionarily conserved throughout the tree of life, and in some cases may play important roles in human disease-causing factors such as the hepatitis C virus, dengue virus and Zika virus.
"These helicases play an unclear role in assembling the hepatitis C virus, for example, but this work suggests that they might be working like a kid sucking on spaghetti to get RNA into the virus particle," Staley said.
"Errors just one nucleotide away from the correct site during splicing can corrupt gene expression and have devastating effects on the cell," he adds. "The implication of our work is that the helicases could be repressed and the pathway of alternative splicing interrupted, so we're very keen on looking for disease-associated mutations in these molecules."
More information: Daniel R. Semlow et al. Spliceosomal DEAH-Box ATPases Remodel Pre-mRNA to Activate Alternative Splice Sites, Cell (2016). DOI: 10.1016/j.cell.2016.01.025
Journal information: Cell
Provided by University of Chicago Medical Center