September 25, 2015 report
Researchers map the main protein entry gateway of mitochondria

(Phys.org)—A team of researchers from Australia, Germany, Japan and Sweden has succeeded in successfully mapping the main protein entryway into mitochondria. In their paper published in the journal Science, the team describes their exhaustive effort which covered a 15 year period.
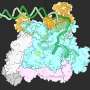
Mitochondria are organelles that are responsible for energy management at the cell level—they also perform regulatory functions and play a part in metabolic pathway control. They are made up mostly of proteins which must be continuously replenished. Precursor proteins are made in the cytosol, the liquid part of cells that hold the organelles. Prior research has shown that there is one main gateway into mitochondria, where those precursors are allowed in—it is called the outer membrane translocator complex, or TOM complex, for short. It is called a complex because it does not just open a door that allows in precursors, it controls which come in and in what amounts. Scientists have wanted to understand how the Tom complex works for many years—a map describing which molecules are allowed in and under which conditions would, for example, allow for the creation of drugs (such as treatments for diabetes) that are allowed into the mitochondria as desired. Creating this map is what the researchers have been steadily working on for the past decade and a half and now they have announced in their paper, that they have finally finished the work.
It was not easy—the team members describe the work as similar to working on a massive Rubik's cube at the nano-scale. It involved using genetically modified yeast probes (as a stand-in for human proteins) and subatomic imaging. The probes when exposed to a burst of UV light would reflect onto other structures in the TOM complex allowing the researchers to gain an understanding of how they were put together. The team would place hundreds of such probes, collect the data and put it all into a computer for processing. The process was carried out over and over, revealing more and more of the structure until a complete map of the entire TOM complex was made.
The researchers note that in addition to the map they created, their technique has proven that it can be used to map entryways into organelles in general, which means it could be used to do the same with virtually all of the organelles that exist in a given cell.
More information: "Molecular architecture of the active mitochondrial protein gate." Science 25 September 2015: Vol. 349 no. 6255 pp. 1544-1548 DOI:10.1126/science.aac6428
ABSTRACT
Mitochondria fulfill central functions in cellular energetics, metabolism, and signaling. The outer membrane translocator complex (the TOM complex) imports most mitochondrial proteins, but its architecture is unknown. Using a cross-linking approach, we mapped the active translocator down to single amino acid residues, revealing different transport paths for preproteins through the Tom40 channel. An N-terminal segment of Tom40 passes from the cytosol through the channel to recruit chaperones from the intermembrane space that guide the transfer of hydrophobic preproteins. The translocator contains three Tom40 β-barrel channels sandwiched between a central α-helical Tom22 receptor cluster and external regulatory Tom proteins. The preprotein-translocating trimeric complex exchanges with a dimeric isoform to assemble new TOM complexes. Dynamic coupling of α-helical receptors, β-barrel channels, and chaperones generates a versatile machinery that transports about 1000 different proteins.
Journal information: Science
© 2015 Phys.org