Building scaffolds in the cell's power stations

A group of scientists led by Assistant Professor Dr. Martin van der Laan has decoded the molecular basis for the characteristic structures inside of mitochondria. Mitochondria are the powerhouses of cells and contain microscopic, strongly infolded membrane structures. These structures allow mitochondria to use the energy gained from food effectively. A defect in the architecture of mitochondrial membrane folds can lead to serious disorders in the nervous and muscular system. The team of researchers from the University of Freiburg has now published a study in the international professional journal Cell Metabolism in which it describes a sophisticated molecular structure made of membrane proteins. This structure allows mitochondria to develop their typical architecture while keeping the elaborate network of membrane folds stable.
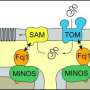
A large complex of several protein components, called the MICOS complex (the acronym for mitochondrial contact site and cristae organizing system), plays a key role in the inner structure of mitochondria and was discovered by the same group of scientists from the University of Freiburg several years ago. For the current study, van der Laan's team worked together with researchers from the University of Groningen in the Netherlands and the Max-Planck-Institute of Biophysics in Frankfurt to decode the blueprint and functions of the MICOS. They determined that Mic10, which is a component of the MICOS, plays a central role. Dr. Maria Bohnert, an expert in molecular medicine and biochemistry from the University of Freiburg, discovered a structure within the protein Mic10 that functions like a barcode. It contains information that describes where Mic10 belongs within the cell. It thus controls the transport of Mic10 and its insertion into the inner membrane system of mitochondria. When the protein has reached its final destination, a second characteristic structure of Mic10 enables it to join many identical copies together to create an extended protein scaffold that keeps the mitochondria's specialized membrane folds together.
If one of the two main structural elements in Mic10 is inactivated, parts of the membrane system collapse, leading to mitochondrial malfunction. On the other hand, if there is too much Mic10, an extreme expansion of the mitochondrial membrane folds occurs. "Our results show that Mic10 is the structural basis of MICOS. It is vital for building the small generators in the cell's power station," Bohnert said. These discoveries could help scientists better understand many different disorders related to the defective construction of mitochondria and the partial loss of mitochondrial functions.
More information: Maria Bohnert, Ralf M. Zerbes, Karen M. Davies, Alexander W. Mühleip, Heike Rampelt, Susanne E. Horvath, Thorina Boenke, Anita Kram, Inge Perschil, Marten Veenhuis, Werner Kühlbrandt, Ida J. van der Klei, Nikolaus Pfanner, and Martin van der Laan: "Central Role of Mic10 in the Mitochondrial Contact Site and Cristae Organizing System", Cell Metabolism, published online 5 May 2015.
Journal information: Cell Metabolism
Provided by BIOSS - Centre for Biological Signalling Studies