A triangular protein pump
Ludwig Maximilian University of Munich researchers have elucidated the structure of a molecular machine with an atypical triangular shape that is involved in peroxisome biogenesis, and characterized its conformation in different functional states.
Peroxisomes are membrane-bound organelles which are involved in a variety of metabolic processes in cells. Of these, the most important are the oxidation of fatty acids and the detoxification of hydrogen peroxide. The enzymes that accomplish these biochemical transformations must be imported into the organelle in a folded form. "It is not entirely clear how this transmembrane transport process works, although it is known that it requires a molecular machine that is made up of two so-called ATPases," says Dr. Petra Wendler, who leads a research group at LMU's Gene Center. In collaboration with Professor Ralf Erdmann's group at the Ruhr- University in Bochum, Wendler's team has determined the molecular structure of the complex isolated from yeast cells in different functional states. The resulting snapshots provide insights into the mode of action of this important transport factor.
ATPases are enzymes that cleave ATP – the basic reaction which supplies metabolic energy for biochemical work in the cell. In this case, the ATPases Pex1 and Pex6 utilize the chemical energy stored in ATP to drive the transport of specific substrates into the interior of the peroxisomes. If either protein is defective, uptake of substrates is impaired. Levels of peroxisome activity are correspondingly reduced and complete loss of the organelles may ensue. Thus, mutations in Pex1 or Pex6 are responsible for most cases of Zellweger syndrome in humans, a rare congenital disease that results in early death.
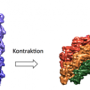
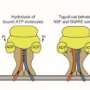
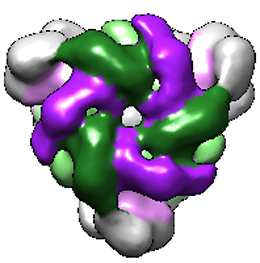
"The complex formed by Pex1 and Pex6 is made up of six subunits, but how the two ATPases are arranged and how they interact with each other was hitherto unknown," Wendler notes. By analyzing samples of the purified complex using electron microscopy, Wendler and her colleagues ascertained that the complex is made up of three Pex1/Pex6 pairs, which are arranged in a ring. The two proteins in each dimer can be distinguished by the fact that the structured domains at their N-terminal ends are asymmetrically oriented. Indeed, to their surprise, the researchers found that viewed from above, the complex is shaped like an equilateral triangle. Similar ATPases involved in the transport of other substrates have been characterized previously but Petra Wendler points out that "none of the hexameric ATPases investigated so far is shaped like this one." By isolating the complex in the presence of various chemical derivatives of ATP, the team was able to visualize the structure of the complex in different conformational and functional states. "These snapshots reveal that hydrolysis of ATP to ADP is accompanied by a pump-like movement of the substrate-binding domains, which strongly suggests that substrates are pulled through the pore in the center of the complex," Wendler explains. The next step will be to characterize the dynamics of the complex, and its interaction with adaptor proteins and substrates, in greater detail.
More information: "Molecular snapshots of the Pex1/6 AAA+ complex in action." Nature Communications 6, Article number: 7331 DOI: 10.1038/ncomms8331
Journal information: Nature Communications
Provided by Ludwig Maximilian University of Munich