June 16, 2014 feature
Introducing synthetic features to living organisms without genetic modification
(Phys.org) —Genetic engineering is one of the great achievements of modern science, allowing for the insertion or deletion of genes in order to control an organism's characteristics and behaviors. However, genetic engineering has its drawbacks, including the difficulties involved in engineering living systems and the potential long-term consequences of altering ecosystems with engineered organisms.
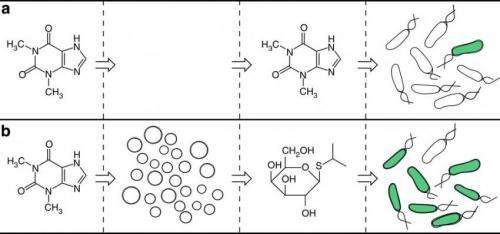
But a new study has shown that controlling organisms on the cellular level does not necessarily require genetic modification. Writing in Nature Communications, Roberta Lentini, et al., have demonstrated that Escherichia coli (E. coli) behavior can be controlled by constructing artificial cells that first sense molecules that E. coli alone cannot sense, and then release different molecules that E. coli can sense. In a way, the artificial cells act as translators by converting unrecognized signals into a chemical language that organisms can understand. The translated signal can then potentially trigger a controllable response in the organism.
"In my opinion, the greatest significance of our work is that it shows that there's more than one way to do synthetic biology," coauthor Sheref Mansy, an assistant professor of biochemistry at the University of Trento in Italy, told Phys.org. "Too often everyone gets excited about one technology or one approach, which sometimes means that solutions to problems get missed because these potential solutions don't depend on prevalent methods. What we've shown is that artificial cells could be used to get around a few of the aspects of living technologies that make people uncomfortable."
In their experiments, the researchers constructed artificial cells that contain a special vesicle which in turn contains several biological components, including a chemical that E. coli can sense (isopropyl b-D-1 thiogalactopyranoside, or IPTG) and DNA that encodes for a riboswitch that responds to an external stimulus. In this case, the external stimulus is the molecule theophylline, commonly found in cocoa beans.
When the artificial cell's riboswitch detects the presence of theophylline, it activates the translation process: a small pore opens in the cell, resulting in the release of IPTG. The E. coli responds to IPTG by exhibiting a green fluorescence, enabling the researchers to easily observe that the new strategy works successfully.
Although E. coli does not respond to theophylline on its own, the artificial cells effectively "expand the senses" of the bacteria by allowing it to indirectly respond to theophylline by translating the chemical message. In this way, E. coli's cellular behavior can be controlled without the need for genetic engineering. The new strategy can potentially overcome the disadvantages of genetic engineering, including the technical difficulties and unintended side effects.
The researchers highlight several examples of how artificial cells may play a role in controlling cellular behavior. One application is using bacteria to search for and clean up environmental contaminants. Instead of genetically engineering bacteria to do this, artificial cells could be constructed to sense the contaminant molecules and release chemoattractants that lure natural bacteria capable of feeding on the contaminants to the site.
Artificial cells could also be used for medical applications, such as to destroy tumors and bacterial infections. For example, rather than spraying engineered bacteria into the lungs of cystic fibrosis patients, artificial cells could be built to detect the presence of specific biofilms, and then release small molecules to disperse the biofilms and thus clear the infection. Similar strategies could also be used to replace engineered probiotics in food and supplements with artificial cells that communicate with gut microbiota to prevent disease.
Before these applications can be realized, however, artificial cells will need several improvements. One of the most important limitations is the batch-to-batch variability of the artificial cells, which results in varying degrees of activity. More work also needs to be done to protect against degradation of the artificial cells' membranes, which would result in the release of the encapsulated molecules even in the absence of the environmental molecules. Future work may also include merging non-genetically modified and genetically modified components to tailor specific cellular features.
"We'd like to make the artificial cells more robust so that they can survive harsher and more varied conditions," Mansy said. "Ultimately we'd like to build artificial cells that can function inside of animals or in the environment, but right now they are probably too fragile."
More information: Roberta Lentini, et al. "Integrating artificial with natural cells to translate chemical messages that direct E. coli behavior." Nature Communications. DOI: 10.1038/ncomms5012
Journal information: Nature , Nature Communications
© 2014 Phys.org