June 27, 2014 report
Researchers find a metastable phase exists throughout transition state of lithium ion batteries
(Phys.org) —A team of researchers with members from the University of Cambridge, Argonne National Laboratory and Stony Brook University have unlocked one of the secrets of lithium ion batteries. In their paper published in the journal Science, the team describes how they used X-ray and powder diffraction to better understand what occurs during the discharge phase of a lithium ion battery and what they found as a result. University of Southhampton chemists John Owen and Andrew Hector offer a Perspective piece on the work by the team in the same journal issue.
Lithium ion batteries have become wildly popular in recent years due to their use in hand-held personal electronic devices and in automobiles—the fast charge/discharge cycling rate make them ideal candidates for use in a wide variety of applications, yet, scientists still don't fully understand how they work. In this new effort, the researchers sought to find out what exactly happens during what most scientists believed was a phase change inside the battery.
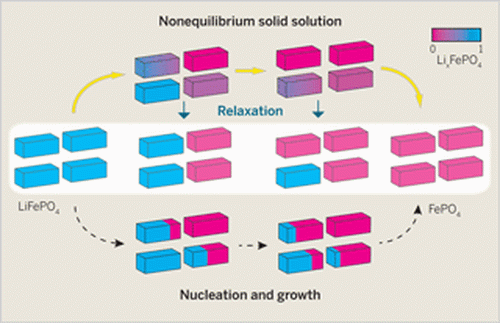
Lithium ion phosphate (LiFePO4) is used in the positive electrode of a lithium ion battery—the conventional understanding is that during the discharge process LiFePO4 undergoes a phase change to FePO4. Now it appears that isn't exactly the case. X-ray diffraction has revealed that phase transformation is actually bypassed during battery cycling as a metastable solid solution comes to exist throughout the electrochemical process. This discovery appears to solve one of the most basic of mysterious surrounding lithium ion batteries—how they manage to have such high performance despite having a miscibility gap and a phase transformation. Such characteristics typically mean slow performance. Now it appears that the miscibility gap is bypassed and there isn't really any phase change after all. That explains how it is that lithium ion batteries are so fast and are reversible for thousands of charge/discharge cycles.
Using X-ray and powder diffraction allowed the researchers to see how diffraction peaks changed during discharge—asymmetric peaks suggested the existence of a phase that existed between the two that were already known—a metastable phase that allows for fast discharging. Owen and Hector suggest the findings could lead the way to use of other materials with similar properties that might help to create batteries that have more or higher power and longer cycle life.
More information: Capturing metastable structures during high-rate cycling of LiFePO4 nanoparticle electrodes, Science 27 June 2014: Vol. 344 no. 6191. DOI: 10.1126/science.1252817
Abstract
The ability to achieve high cycling rates in a lithium-ion battery is limited by the Li transport within the electrolyte; the transport of Li ions and electrons within the electrodes; and, when a phase transformation is induced as a result of the Li compositional changes within an electrode, the nucleation and growth of the second phase. The absence of a phase transformation involving substantial structural rearrangements and large volume changes is generally considered to be key for achieving high rates. This assumption has been challenged by the discovery that some nanoparticulate electrode materials, most notably LiFePO4, can be cycled in a battery at very high rates, even though they cycle between two phases during battery operation. This apparent contradiction has been reconciled by the hypothesis that a nonequilibrium solid solution can be formed during reaction to bypass the nucleation step.
Perspective: Phase-transforming electrodes, www.sciencemag.org/content/344/6191/1451
Journal information: Science
© 2014 Phys.org