March 6, 2014 report
Researchers discover new group of quasicrystals

(Phys.org) —A team of researchers working at the university of Notre Dame has discovered a whole new group of quasicrystals. In their paper published in the journal Nature, the team describes how they accidently created a new kind of quasicrystal as part of a series of experiments designed to learn more about electron distribution in ferrocenecarboxylic acids.
Quasicrystals are groups of molecules bonded together in structures that resemble crystals in that they are organized, but unlike crystals, the structures are not nearly as uniform. In fact, they are quite the opposite—though they are locally symmetric, they lack any sort of long distance periodicity. Because of their chaotic nature, quasicrystals tend to feel slippery to the touch, which is why they have been used to coat the surface of non-stick frying pans. The first quasicrystal was made, also by accident, in 1982, by Daniel Shechtman (who later won a Nobel prize for his work). Since then many more of them have been made in various labs, (one was even found to exist in a meteorite) though most of them have had one thing in common, they were all formed from two or three metal alloys.
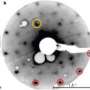
In this latest discovery, the quasicrystals self-formed after the researchers placed a layer of iron containing molecules of ferrocenecarboxylic acid on top of a gold surface. The team was expecting to see a linear group of stable molecules pairing up as dimers, but instead were surprised to find that they had formed into five sided rosettes—it was the rosettes that pushed other molecules into bonding forming crystalline shapes, resulting in the formation of 2D quasicrystals that took the form of several different shapes: stars, boats, pentagons, rhombi, etc., all repeated in haphazard fashion.
In studying the quasicrystals using scanning tunnelling microscopy, the researchers found that they were held together by weak hydrogen bonds rather that the strong ionic bonds found in other such molecules. Weak hydrogen bonds are generally more common in organic molecules that exhibit complex structures.
In their paper, the researchers suggest their discovery might lead to the creation or discovery of many other similar types of quasicrystals, though it's still not clear to what use they might be put.
More information: Self-assembly of hydrogen-bonded two-dimensional quasicrystals, Nature 507, 86–89 (06 March 2014) DOI: 10.1038/nature12993
Abstract
The process of molecular self-assembly on solid surfaces is essentially one of crystallization in two dimensions, and the structures that result depend on the interplay between intermolecular forces and the interaction between adsorbates and the underlying substrate1. Because a single hydrogen bond typically has an energy between 15 and 35 kilojoules per mole, hydrogen bonding can be a strong driver of molecular assembly; this is apparent from the dominant role of hydrogen bonding in nucleic-acid base pairing, as well as in the secondary structure of proteins. Carboxylic acid functional groups, which provide two hydrogen bonds, are particularly promising and reliable in creating and maintaining surface order, and self-assembled monolayers of benzoic acids produce structure that depends on the number and relative placement of carboxylic acid groups2, 3, 4, 5, 6. Here we use scanning tunnelling microscopy to study self-assembled monolayers of ferrocenecarboxylic acid (FcCOOH), and find that, rather than producing dimeric or linear structures typical of carboxylic acids, FcCOOH forms highly unusual cyclic hydrogen-bonded pentamers, which combine with simultaneously formed FcCOOH dimers to form two-dimensional quasicrystallites that exhibit local five-fold symmetry and maintain translational and rotational order (without periodicity) for distances of more than 400 ångströms.
Journal information: Nature
© 2014 Phys.org