A promising new system for cheaper drug preparation
Researchers at the Institute of Chemical Technology (ITQ), a joint centre of the Universitat Politècnica de València and the Spanish National Research Council (CSIC), and the Delft University of Technology (Netherlands) have developed a new photobiocatalytic system that could imply substantial changes in the way active molecules are prepared for drugs and other catalysis-based chemical products. The system uses enzymes as catalysts, water as a supplier of electrons and light as an energy source. The research results developed by the Spanish and Dutch scientists have been published in the journal Nature Communications.
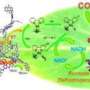
The feasibility of photobiocatalytic, water-driven bioredox reactions is demonstrated for the first time. The thermodynamic driving force required for water oxidation is obtained from UV and visible light by means of simple titanium dioxide-based photocatalysts. The new combination of an enzyme, light (UV and visible spectrum), photocatalyst and water as an electron source for biocatalysis pave the way to possible technological alternatives for the enzymatic production of new drugs and the pharmacy industry in generally, pesticides and other fine chemicals. The project leaders are Avelino Corma (ITQ) and Frank Hollmann (Department of Biotechnology, Delft University of Technology).
In this process, the Spanish and Dutch researchers have used enzymes to perform enantioselective reactions. As Professor Corma says, enzymes are catalysts that act on living organisms, "you could say that thanks to them life on earth as we know it is possible." Some of these enzymes are isolated and can be used to catalyse chemical reactions at an industrial level. However, there are enzymes, such as oxidoreductases, that are highly interesting to the industry but are not used because of the cost of the process. This is because the oxidoreductases require for their catalytic function high cost molecules, called cofactors, which act as an intermediary in the process and are deactivated.
"To reactivate and maintain their functioning, other cofactors and enzymes are needed, leading to very complex systems too difficult for industrial application. The system can be simplified by using a cosubstrate (another usually high-cost molecule) that allows cofactor regeneration. However, the cosubstrate loses its ability to react and becomes a reaction residue. This means that for every molecule of desired product generated there is a deactivated cosubstrate molecule produced as waste, with the consequent financial loss." This article shows that it is possible to attach a photocatalytic system with an enzymatic process, obtaining what is called a photobiocatalytic system for which the cosubstrate is simply water.
"In other words, with water, light and a semiconductor, we can generate the electrons and protons needed for the oxidoreductase enzyme to act and hydrogenate molecules by an enantioselective reaction. This new concept and photobiocatalytic system open the possibility of enzymatic processes which were previously limited by the cost of the cofactor, such as oxidoreductases, suddenly becoming economically viable," states Avelino Corma.
Sara Iborra, coauthor of the article and researcher of the ITQ adds another of the keys of the study: "the cofactor of our catalysis—in this case the flavin mononucleotide involved in the reaction—takes an electron from the oxidation of the water caused by light and the photocatalyst, and transfers it to the enzyme, which in turn triggers and catalyses the chemical reaction. The process is regenerated and repeats itself again and again. The thermodynamic energy required for the reaction is obtained from the light. And all this takes place at room temperature. Moreover, by obtaining the energy from light and from the electrons of water, the process is performed without producing waste and is a very simple alternative to the extremely complicated methods used today for regenerating enzyme cofactors that involve electron transfer. These are the most important advantages of the new process we have developed."
As the researchers explain, in many processes of organic synthesis, a mixture of two isomers (substances that, with the same chemical composition have different properties) is obtained. These isomers (called enantiomers) differ only in that the structure of one is a mirror image of the other. Therefore, they differ only in the spatial arrangement of atoms and are symmetrically equivalent but different molecules, as in the case of a person's hands.
"Though these isomers are different compounds and can have very different biological activities, their physical and chemical properties are identical, and to separate them by physical or chemical methods is expensive," adds María Mifsud, also coauthor and researcher of the ITQ.
In the pharmaceutical industry, for example, is very important to obtain one enantiomer or the other, and in many cases enantioselective catalysts that only produce the desired isomer are used. "For example, the active principle which is used to treat Parkinson's disease, L- dopa, is one of the two enantiomers, but it is the one that produces the beneficial effect of inhibiting the disease. The other enantiomer, its symmetrical, is toxic and produces the opposite effect. This gives an idea of how important it is to obtain the correct substance in these processes and that is why enantioselective catalysts are used," says Maria Mifsud.
More information: María Mifsud, Serena Gargiulo, Sara Iborra, Isabel W.C.E. Arends, Frank Hollmann y Avelino Corma. Photobiocatalytic chemistry of oxidoreductases using water as the electron donor. Nature Communications. DOI: 10.1038/ncomms4145
Journal information: Nature Communications
Provided by Asociacion RUVID