Researchers open new possibilities for characterizing nanoparticle interactions
(Phys.org) —Molecules anchored to the surfaces of nanoparticles modify and even control many characteristics of the particles, including how they interact with cells or react to light. The type of binding affects the nanoparticle's behavior and interaction with surrounding particles, atoms and molecules. Unfortunately, methods to directly study the surface bonding at a nanoparticle solid/liquid interface have been elusive, as the interface is usually not accessible by most existing techniques. Researchers at EMSL took advantage of advanced instrumental capabilities, a specially designed experimental cell and theoretical modeling to successfully deduce the how molecules of carboxylic acid– a common organic acid found in nature - bind to ceria nanoparticle surfaces.
Because of this research, the scientific community now has a new and potentially powerful way to characterize the interactions of nanoparticles with molecules in a range of environments, possibly extending to their behaviors in living cells. As such "hidden" interfaces are common in nature and in our bodies, characterizing and understanding the structure and interactions where liquids and solids meet can accelerate design of new molecules to solve problems in medicine, environmental remediation, climate studies, biofuels, catalysts and energy storage.
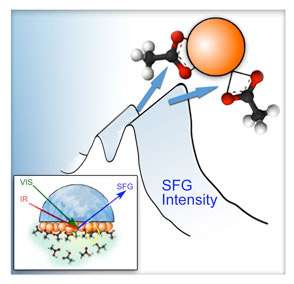
The experimental work was performed on EMSL's sum-frequency generation vibrational spectrometer (SFG-VS). SFG-VS is a sensitive optical spectroscopy that can selectively measure vibrational spectra of molecules bonded on surfaces with the "fingerprint" frequencies to help determine the bonding species and their structures. Because of experimental challenges and the difficulty of interpretation without theoretical guidance, SFG has not usually been extended to probe nanoparticle surfaces buried in liquid. At EMSL, an optical cell was designed to perform in-situ experiments using a CaF2 window (which can transmit infrared light) deposited with ceria nanoparticles in contact with acetic acid solution. SFG-VS identified resonant frequencies of the vibrations of molecular bonds between the acid and the ceria surfaces in different oxidation states.
Theoretical modeling was crucial to the successful identification of the bonds. First-principles theory was used to predict the stable structures by modeling various ways acetic acid can bind to surface sites on ceria clusters. The resonant frequencies of thermodynamically favored structures were calculated to compare with those from the SFG-VS experiment. Modeling results revealed that acetic acid binds differently on reduced surfaces of ceria than on oxidized surfaces, in accord with experimental results.
More information: Z. Lu, A. S. Karakoti, L. Velarde, W. Wang, P. Yang, S. Thevuthasan, and H. Wang, "Dissociative binding of carboxylic acid ligand on nanoceria surface in aqueous solution: A joint in situ characterization and first-principles study." J. Phys. Chem. C 117, 24329, 2013. dx.doi.org/10.1021/jp4068747.
Provided by Environmental Molecular Sciences Laboratory