Oxygen 'sponge' presents path to better catalysts, energy materials
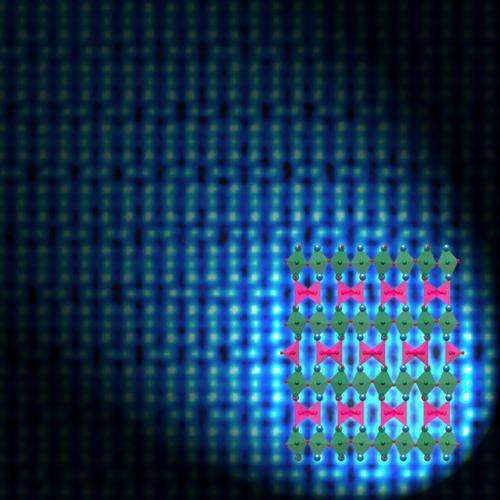
Scientists at the Department of Energy's Oak Ridge National Laboratory have developed a new oxygen "sponge" that can easily absorb or shed oxygen atoms at low temperatures. Materials with these novel characteristics would be useful in devices such as rechargeable batteries, sensors, gas converters and fuel cells.
Materials containing atoms that can switch back and forth between multiple oxidation states are technologically important but very rare in nature, says ORNL's Ho Nyung Lee, who led the international research team that published its findings in Nature Materials.
"Typically, most elements have a stable oxidation state, and they want to stay there," Lee said. "So far there aren't many known materials in which atoms are easily convertible between different valence states. We've found a chemical substance that can reversibly change between phases at rather low temperatures without deteriorating, which is a very intriguing phenomenon."
Many energy storage and sensor devices rely on this valence-switching trick, known as a reduction-oxidation or "redox" reaction. For instance, catalytic gas converters use platinum-based metals to transform harmful emissions such as carbon monoxide into nontoxic gases by adding oxygen. Less expensive oxide-based alternatives to platinum usually require very high temperatures—at least 600 to 700 degrees Celsius—to trigger the redox reactions, making such materials impractical in conventional applications.
"We show that our multivalent oxygen sponges can undergo such a redox process at as low as 200 degrees Celsius, which is comparable to the working temperature of noble metal catalysts," Lee said. "Granted, our material is not coming to your car tomorrow, but this discovery shows that multivalent oxides can play a pivotal role in future energy technologies."
The team's material consists of strontium cobaltite, which is known to occur in a preferred crystalline form called brownmillerite. Through an epitaxial stabilization process, the ORNL-led team discovered a new recipe to synthesize the material in a more desirable phase known as perovskite. The researchers have filed an invention disclosure on their findings.
"These two phases have very distinct physical properties," Lee said. "One is a metal, the other is an insulator. One responds to magnetic fields, the other does not—and we can make it switch back and forth within a second at significantly reduced temperatures."
The international team's design and testing of this novel advanced material from scratch required multidisciplinary expertise and sophisticated tools from such places as Argonne National Laboratory and ORNL, including Argonne's Advanced Photon Source and ORNL's Center for Nanophase Materials Science, says Lee.
"As we showed in this study, only through the study of a well-defined system can we build a framework for the design of next generation energy materials," said coauthor John Freeland of Argonne. "This insight was made possible by merging the capabilities at Oak Ridge and Argonne national labs for advanced synthesis and characterization of novel materials."
More information: "Reversible redox reactions in an epitaxially stabilized SrCoOx oxygen sponge," Nature Materials.
Journal information: Nature Materials
Provided by Oak Ridge National Laboratory



















