August 8, 2011 report
Droplets levitating above a liquid surface show unusual motion (w/ video)
When drops of water are sprinkled on an extremely hot skillet, the drops can slide around the skillet for a full minute or so before evaporating. The phenomenon is called the Leidenfrost effect, which says that when a surface is significantly hotter than the boiling point of a given liquid (the “Leidenfrost point”), droplets of that liquid will take longer to evaporate than if the temperature of the surface were somewhat cooler - above the liquid’s boiling point but below the Leidenfrost point. (For water, the Leidenfrost point is 250 °C [482 °F].)
The effect occurs because the bottom part of the droplet immediately vaporizes on contact with the hot surface, and the resulting gas suspends the droplet above the surface. No longer touching the hot surface, the droplet evaporates more slowly and, due to reduced friction, can slide around on the thin layer of gas.
In a new study to be published in PNAS, Marie Le Merrer from CNRS-Ecole Polytechnique in Palaiseau, France, and CNRS-ESPCI-Paris, and her coauthors have investigated the cause of the deceleration of liquid nitrogen drops sliding in a Leidenfrost state just above a liquid surface. Unlike typical Leidenfrost drops on hot surfaces, these Leidenfrost drops exist at room temperature.
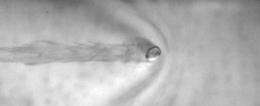
In their experiments, the researchers carefully threw millimeter-sized liquid nitrogen drops at a certain velocity tangent to the surface of water or silicone oil. Then they recorded the drops’ motion with a high-speed video camera from above. Although the drops took about a minute to evaporate, the researchers were interested only in the first second of the drops’ motion. They found that the drops on water slowed down significantly between 0.05 and 0.18 seconds, with a deceleration of about 170 cm/s2.
This deceleration of drops on water is much higher than it is on a solid surface (where deceleration is about 10 cm/s2), suggesting that the friction on water is about 10-100 times stronger than the friction on a solid surface. When analyzing their video, the researchers found that the increased friction on water is due primarily to wave resistance. A drop moving above the water’s surface can generate waves, whose wake the researchers observed in the videos.
When performing the same experiment on the more viscous oil, the researchers found that the deceleration is much lower than it is on the water, and they did not observe waves as they did on the water. These results further support the idea that wave resistance is the main contributor to the deceleration of Leidenfrost drops on water.
The study helps to clarify wave resistance, which occurs in many situations but is usually difficult to measure since friction on an object moving at the water’s surface is the sum of different contributions, from which it is difficult to extract the sole wave resistance. By using floating bodies that do not penetrate water or oil, the researchers could ensure that the main source of resistance was wave resistance. Understanding wave resistance could have a wide variety of implications, such as providing a clearer picture of the motion of insects at the surface of water ponds.
More information: Marie Le Merrer, et al. PNAS. To be published.
© 2010 PhysOrg.com