Ordered Water: Just how much water is there in calcined gypsum?
(PhysOrg.com) -- Gypsum was used as a building material in antiquity and is still widely used as a binder in plaster, drywall, and spackling paste. Known as dihydrate in construction chemistry, gypsum is a water-containing calcium sulfate (CaSO4• 2 H2O). In various calcination processes, some of the water of crystallization is removed, resulting in calcined gypsum, or hemihydrate (CaSO4• 0.5 H2O). When this material comes into contact with water, it reabsorbs it and sets up. The structure and exact water content of hemihydrate have remained a matter of speculation.
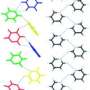
Michael F. Bräu (BASF Construction Chemicals GmbH) and Horst Weiss (BASF SE) have now brought this speculation to an end: by using single-crystal structural analyses they were able to solve the structure, generate a structural model, and support it with computer calculations. As reported in the journal Angewandte Chemie, hemihydrate does indeed contain exactly one half of a water molecule per structural unit -- tightly bound to the calcium sulfate framework.
Hemihydrate is the most heavily produced inorganic compound worldwide, so its structure and water content are of great interest, both economically and scientifically. The first structural model of this compound was proposed in 1933, and it still holds today. Since then, there have been a number of refined models, which do a good job of reproducing the fundamental calcium sulfate scaffold. However, there has always been disagreement about whether the water molecules also adopt a defined arrangement and if so, what it looks like.
Answering such questions requires structural analyses based on X-ray diffraction experiments carried out on single crystals of the right size and quality. The atoms of a crystal deflect incoming X-rays; the resulting characteristic diffraction pattern makes it possible to calculate the positions of the individual atoms in the crystal. However, this has been very difficult to achieve in the case of gypsum crystals. Bräu and Weiss have now been successful. By using various tricks they were able to interpret the diffraction pattern and to use their computer calculations to consolidate the data into a plausible structural model.
The alignments of the individual water molecules and their distances from each other prove that there are no interactions between them; they are bound only to the calcium sulfate framework. They are packed in so tightly that no further water molecules can enter into the channels of the basic structure. Variations of the crystal with a proportion of water molecules above 0.5 per formula unit thus seem to be very unlikely.
More information: Michael F. Bräu, How Much Water Does Calcined Gypsum Contain? Angewandte Chemie International Edition 2009, 48, No. 19, 3520-3524, doi: 10.1002/anie.200900726
Provided by Wiley (news : web)