Discovery could help scientists stop the "death cascade" of neurons after a stroke
(PhysOrg.com) -- Distressed swimmers often panic, sapping the strength they need to keep their heads above water until help arrives. When desperate for oxygen, neurons behave in a similar way. They freak out, stupidly discharging energy until they drown in a sea of their own extruded salts. Every year, millions of victims of stroke or brain trauma suffer permanent brain damage because of this mad rush to oblivion that begins once a part of the brain is deprived of blood.
It is well known that a ubiquitous cell receptor drives these oxygen-starved neurons’ lemming-like behavior. But this particular receptor, for the neurotransmitter glutamate, is also responsible for the rapid transmission of information between neurons required for all cognition, among other things. Shutting it off has serious consequences, like coma. Now, a team of scientists at The Rockefeller University has identified a single subunit of this receptor that drives neuronal death. This new discovery suggests that drugs targeting a specific subunit of the complex glutamate receptor might be able to slow brain damage without disrupting other crucial brain functions.
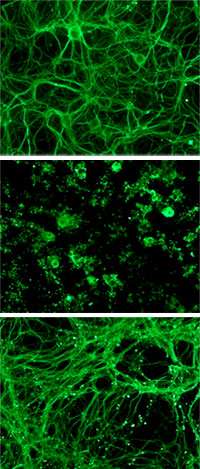
The neuronal panic that occurs when a clot or other insult blocks the flow of blood to part of the brain is called excitotoxic neurodegeneration. It results in the brain cells spitting out glutamate, which then accumulates in the synapses between neurons and stimulates the release of more glutamate. It’s a vicious cycle that kills the cells quickly and continues until blood flow is restored. Doctors often treat stroke victims by administering a heavy dose of a clot-buster called tissue plasminogen activator (tPA), a protein that can stimulate the dissolution of clots. Ironically, however, the same drug that does this crucial clot-busting also accelerates the panicky process that kills neurons, research by Strickland and others has shown. Investigating exactly how tPA does that is what led Strickland’s team to the recent discovery.
Neurons are typically couched in laminin, an extracellular matrix protein known to be involved with tPA in the neuronal “death cascade.” The Strickland lab’s experiments, published in The Journal of Cell Biology, show that tPA produces an enzyme that degrades laminin into toxic products that kill the neurons in their midst, specifically by stimulating the production of one of five subunits for a particular kind of glutamate receptor. The overproduction of this specific subunit, KA1, makes the cells hypersensitive to glutamate, which fans the glutamate frenzy leading to their death.
To better understand the process, Zu-Lin Chen and other colleagues at Rockefeller in the Strickland lab and at the University of Leicester in England designed lines of genetically modified mice that lacked either tPA or laminin specifically in the hippocampus, a region of the brain often damaged by stroke. To their surprise, they found that mice without laminin were protected from the typical neural degeneration that follows a simulated stroke in regular mice. They also found that mice with laminin but not tPA were relatively protected.
But when they injected degraded laminin into mouse brains without laminin or tPA they found a similar overproduction of the subunit of the glutamate receptor that they measured when inducing stroke in normal mice. The problem: Laminin, once degraded by tPA, prompts the proliferation of the receptor subunit that makes the cells suicidally sensitive to glutamate. By preventively injecting a molecule that disables that particular subunit, they were able to dramatically reduce the cell death following a stroke. A big plus: The treated mice did not suffer the severe side effects that come with blocking the entire glutamate receptor.
Whether this will turn into a therapy that can be applied after a stroke is uncertain.
“Can you do it after the fact? That will be a question,” Strickland says. “Cell death happens pretty quickly. But it’s an interesting avenue to pursue.”
Reference: The Journal of Cell Biology 183(7): 1299-1313 (December 29, 2008), jcb.rupress.org/cgi/content/abstract/183/7/1299
Provided by Rockefeller University