Watching Catalytic Reactions from Within
(PhysOrg.com) -- Researchers from Utrecht University, in The Netherlands, have demonstrated a new way to get a real-time, microscopic view of the inner workings of catalytic reactions.
Catalysis, a natural and manmade way to accelerate and direct chemical reactions, is a common process used in everything from the production of gasoline, electronic devices, and clothing, to the development of clean and renewable energy technologies. Traditionally, catalysts are studied using a synchrotron technique called x-ray absorption spectroscopy (XAFS), during which samples are bombarded with beams of high-energy x-rays. Because every compound and element on Earth absorbs x-rays differently, producing a unique "signature," researchers can use XAFS to follow sample's transformation during the catalytic processes.
But XAFS is not always capable of giving clear details about the chemical transformation that reactant molecules undergo on the catalyst's surface.
"Catalysis is the workhorse of modern chemistry yet our understanding of catalytic processes is somewhat limited," said Utrecht chemist Eli Stavitski. "We need to take a look inside of these molecules in order to get a better idea of what's going on."
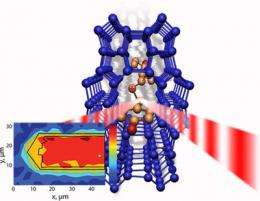
Stavitski and his colleagues found a way to do that at NSLS beamline U10B, where an infrared spectrometer is coupled with an optical microscope to create a powerful technique called infrared microspectroscopy.
"IR microspectroscopy offers unprecedented chemical sensitivity and spatial resolution and gives you direct insight into the chemical nature of the reactant molecules, the potential intermediates, and the resulting products as the catalytic reaction occurs," Stavitski said.
The method demonstrated by the Utrecht group is one of just a few techniques capable of analyzing the internal surfaces of catalyst materials during the course of a reaction, and it's the first to be done using infrared light. The researchers tested the technique with a zeolite - a powerful catalyst used in the petrochemical industry that contains aluminum, silicon, and oxygen. Their results are published and featured on the cover of the April 28, 2008 edition of Angewandte Chemie.
Specifically, the researchers used this new setup to look at the transformation of the organic compound, styrene, within the zeolite crystals. They were able to determine the principal reaction intermediate and identify important structure-function relationships within the material.
One downside to the group's method is limited spatial resolution. With the current IR microspectroscopy setup, the researchers cannot study areas of the catalyst that are larger than the wavelength of the light used - about 3-8 microns (a single human hair, by comparison, is about 100 microns wide). To provide a big-picture view of the catalyst, the Utrecht scientists are looking for ways to improve this characteristic. The group also is trying to expand the technique's capabilities to include the analysis of gas-phase reactions, which are very important to industrial applications.
In addition to Stavitski, other authors on the paper include Marianne Kox, Ingmar Swart, Frank de Groot, and Bert Weckhuysen, all of the Utrecht University Department of Chemistry.
Reference: E. Stavitski, M.H. Kox, I. Swart, F.M.F. de Groot, B.M. Weckhuysen, "In Situ Synchrotron-Based IR Microspectroscopy To Study Catalytic Reactions in Zeolite Crystals," Angew. Chem., 47(19) 3543-3547, (2008).
Provided by Brookhaven National Laboratory