A Biomolecule as a Light Switch
Switchable fluorescent proteins - able to switch themselves reversibly back-and-forth between an "on" and "off" state - have been known for only a few years. However, they already hold promise for a large number of novel applications, from cellular biology to data storage. Cell biologists, X-Ray crystallographers, photobiophysicists, and computer-biophysicists from Goettingen have worked together on a project uncovering the molecular mechanism by which a fluorescent protein becomes switched.
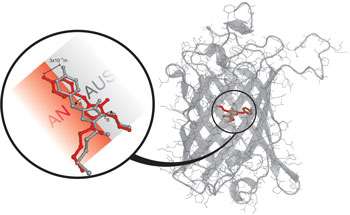
Image: The switch mechanism of a molecular light switch. On the right, the structure of the reversible switchable fluorescent protein asFP595. On the left, in detail, the structure of the chromophore: red for the fluorescent "on" state and gray for the non-fluorescent "off" state. With both green and blue light, the chromophore can be switched back-and-forth between these two states.
This knowledge could be of importance for, among other purposes, optical data storage in protein crystals.
The fluorescent protein identified as asFP595 is found on the ends of the tentacles of the snakelocks anemone Anemonia sulcata, a type of coral which lives in the Mediterranean Sea and North Atlantic, in the areas near the surface of the water, which are flushed with light . In the tentacle ends, this protein probably protects the anemone’s tissue from solar rays that are too strong. asFP595 absorbs green light and eventually emits red fluorescent light. When another light is applied to it, the protein can be switched back-and-forth between a fluorescent and non-fluorescent state. It is a so called "molecular light switch."
The researchers from Goettingen have uncovered the mechanism behind this molecular switch. They fabricated the protein in bacteria, and then, from the purified protein, cultivated crystals that still had the switching characteristics of the free protein. X-ray structural analysis and computer simulations showed that the chromophore - the part of the protein that absorbs the light - changes structure when it is lit up using a cis-trans isomerisation. The chromophore does what is called a "hula twist", changing its position merely 3x10-10 m - a third of a billionth of a meter. This tiny change is enough to turn the fluorescent protein into a non-fluorescent one.
Based on this knowledge, the researchers want to hone the protein with the goal of using it in various applications. They range from highest-resolution microscopy all the way to optical data storage in protein crystals.