Researchers Synthesize Brightest Fluorescent Particles Ever
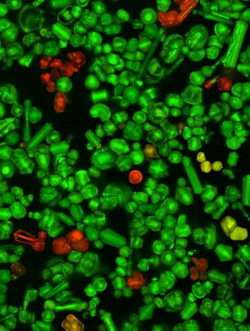
Clarkson University Physics Professor Igor Sokolov and his team have discovered a method of making the brightest ever synthesized fluorescent silica particles.
These nanostructured macroscopic silica particles have potential applications in medicine, forensic science and environmental protection, among many other uses. Sokolov's research is published in the March 5 issue of the scientific journal Small.
Sokolov, along with Ph.D. student Yaroslav Y. Kievsky (now a research fellow at the National Research Council of Canada) and Clarkson undergraduate student Jason M. Kaszpurenko, has created a process to physically entrap a large number of organic fluorescent molecules inside a nanoporous silica matrix. The fluorescence of these particles is 170 times brighter than any particles of similar size created so far. The previous record was reached using quantum dots.
In fluorescence, an initial ignition light energizes molecules, then the molecules reemit the light with a different color. This phenomenon can be used in many different applications because it is easily detectable, using optical filters to remove the ignition light, leaving only the particles' light visible.
There are a multitude of applications for these microscopic "flashlights." These particles, which are less than 1/10th the size of a human hair, could be used like holograms to prove a product's authenticity, imbedded in various objects or fabric for tracking, or used like an "invisible ink" to prove that someone touched an object.
"You could use them like a UPC barcode, encoded and deciphered not in terms of light, but in terms of colors," says Sokolov. "Using only commercially available dyes, you could create and track about 100 trillion combinations of these 'UPC' color barcodes. Because these particles are so bright, it is possible to detect even a single colored particle easily."
There are also potential applications in environmental protection -- dispersing the particles into groundwater to see where it flows to or tracking sources of air pollution. "You could spray these particles into the air like dust," says Sokolov, "and easily collect them because they are so highly visible."
Sokolov sees an ultra-bright future for these fluorescent particles. Down the road he envisions particles that can change color in different acidities. "We have already patented particles that can change color because of temperature change," he says. "The next step is to create a particle that would be a whole laboratory, simultaneously detecting many chemical environment factors -- temperature, acidity, metal ions, etc.
"After that, we could make a nanobot, or smart drug, that would actively react to its environment, and be used, for example, in fighting cancer. Tumor tissue has a higher acidity than the surrounding tissue. As soon as the injected nanobot encountered the high acidity of a tumor, it would start releasing the drug it carries to kill the cancer."
Apart from a family of various sensors, Sokolov's team is now working on scaling down the size of these particles to nanosize (a thousand times smaller than now). "This should have a significant impact in biology," says Sokolov. "For example, you can create particles of different colors. These particles can be made 'sticky' to particular biological molecules inside cells. Then you can see those molecules easily. This fluorescent labeling helps to identify diseased cells and may show exactly what is causing the disease."
Sokolov works on these projects together with postdoctoral fellow Sajo P. Naik and graduate student Dmitry Volkov. Two undergraduate students, Jason M. Kaszpurenko and James O. Benson, both seniors majoring in physics, are also part of the team. "At Clarkson, there are opportunities for undergraduate students in frontier science work like this," says Sokolov. "They are more than welcome to come and work with us."
In fact, Kaszpurenko is a coauthor of the Small journal article and a co-inventor of a patent. Due in part to this undergraduate research experience, Kaszpurenko has been accepted for graduate work at UC Davis, where he will continue his physics studies.
You can see the full article at www3.interscience.wiley.com/cg … /114088575/HTMLSTART .
Source: Clarkson University





















