Learning how nature splits water
About 3.2 billion years ago, primitive bacteria developed a way to harness sunlight to split water molecules into protons, electrons and oxygen, the cornerstone of photosynthesis that led to atmospheric oxygen and more complex forms of life -- in other words, the world and life as we know it.
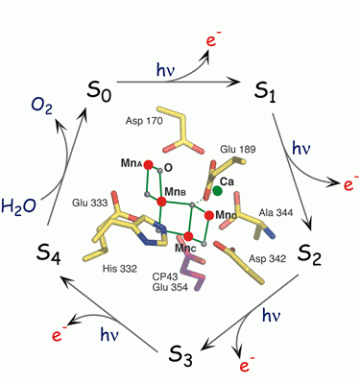
Today, scientists have taken a major step toward understanding this process by deriving the precise structure of a catalyst composed of four manganese atoms and one calcium atom that drives this water-splitting reaction. Their work, detailed in the Nov. 3, 2006 issue of the journal Science, could help researchers synthesize molecules that mimic this catalyst, which is a central focus in the push to develop clean energy technologies that rely on sunlight to split water and form hydrogen to feed fuel cells or other non-polluting power sources.
Specifically, an international team led by scientists from the U.S. Department of Energy's Lawrence Berkeley National Laboratory pieced together high-resolution (approximately 0.15 Ångstrom) structures of a Mn4Ca cluster found in a photosynthetic protein complex (one Еngstrom equals one ten-billionth of a meter). The team, which includes scientists from Germany's Technical and Free Universities in Berlin, the Max Planck Institute in Mülheim, and the Stanford Synchrotron Radiation Laboratory, used an innovative combination of x-ray spectroscopy and protein crystallography to yield the highest-resolution structures yet of the metal catalyst.
"This is the first study to combine x-ray absorption spectroscopy and crystallography in such a detailed manner to determine the structure of an active metal site in a protein, especially something as complicated as the photosynthetic Mn4Ca cluster," says Junko Yano of Berkeley Lab's Physical Biosciences Division, who is one of the lead authors of the study.
The metal catalyst resides in a large protein complex, called photosystem II, found in plants, green algae, and cyanobacteria. The system drives one of nature's most efficient oxidizing reactions by using light energy to split water into oxygen, protons, and electrons. Because of its efficiency and reliance on nothing more than the sun, the catalyst has become a target of scientists working to develop carbon-neutral sources of energy. Learn the catalyst's structure, then how it works, and perhaps scientists can develop similarly robust molecules.
But until now, the precise structure of the catalyst has eluded all attempts of determination by x-ray diffraction and various spectroscopic techniques. Even a 3.0-Ångstrom-resolution structure obtained by the Berkeley Lab group's collaborators at the Technical and Free Universities in Berlin, using x-ray diffraction, didn't allow the researchers to pinpoint the exact positions of the cluster's manganese and calcium atoms and its surrounding ligands. Part of the problem is the fact that the metal catalyst is highly susceptible to radiation damage, which rules out extremely high-resolution x-ray diffraction studies.
To minimize radiation damage, Yano and colleagues combined x-ray absorption fine structure spectroscopy measurements with x-ray diffraction data from crystallographic studies, which were obtained at the Stanford Synchrotron Radiation Laboratory, where the techniques used in this study were developed in collaboration with the Berkeley Lab scientists. This technique exposes the Mn4Ca cluster to much lower doses of radiation, and enabled the team to obtain three similar structures at a resolution much higher than previously possible.
These three structures shed new light on how the catalyst fits within the much larger photosystem II protein complex. The x-ray diffraction structures at a medium resolution are sufficient to determine the overall shape and placement of the catalyst within the protein complex, and the spectroscopy measurements provide high-resolution information about the distances and orientation of the catalyst.
"We have a real structure now," adds Vittal Yachandra, also with Berkeley Lab's Physical Biosciences Division and a co-author of the paper. "It's not just guesswork anymore. Before, there were a lot of disparate pieces and scientists were forced to speculate on the catalyst's structure. Now, we can begin to infer how the energy of sunlight is used to oxidize water to molecular oxygen."
Scientists already know that the catalyst goes through four steps as it oxidizes water to oxygen, with each step triggered by the absorption of a photon. Now, they can learn how individual bonds are broken and formed, and how the water molecule splits apart, step by step. The group's high-resolution structure is already yielding clues.
"We found that our structure is unlike the 3.0-Ångstrom-resolution x-ray structure and other previously proposed models," says Yano. "The higher-resolution structures are likely to be important in gaining a mechanistic understanding of water oxidation."
Ultimately, this research will inform the search for renewable energy sources. Many of the strategies scientists propose depend on a way to wrest hydrogen, which is an energy carrier, from water. Unfortunately, the current methods used to extract hydrogen from water require either electricity or methane, both of which come at a price.
"That's why the water-splitting complex in photosynthesis is the basis for a lot of work being done in energy research today," says Yachandra. "This is the main underpinning for our work. We are trying to understand how nature works so we can apply the same principles to clean energy research."
The Science paper is entitled Where Water is Oxidized to Dioxygen: Structure of the Photosynthetic Mn4Ca Cluster. Co-authoring the paper with Yano and Yachandra are Ken Sauer and Yulia Pushkar from Berkeley Lab and UC Berkeley; Jan Kern and Athina Zouni from the Technical University in Berlin; Johannes Messinger from the Max Planck Institute in Mülheim; Jacek Biesiadka, Bernhard Loll and Wolfram Saenger from the Free University in Berlin; and Matthew Latimer from SSRL.
Source: Lawrence Berkeley National Laboratory