Controversy-plagued superheavy element 118 finally created
Element 118 has been indirectly discovered in experiments conducted at the Flerov Laboratory of Nuclear Reactions in Dubna, Russia by a collaboration of researchers from Russia's Joint Institute for Nuclear Research and the Lawrence Livermore National Laboratory in California.
Researchers at the Lawrence Berkeley National Laboratory previously reported the synthesis of element 118 in 1999, and later retracted their results when subsequent experiments failed to confirm their discovery. Investigations revealed that evidence supporting the production of three atoms of element 118 had been fabricated by one of the key LBNL researchers.
In experiments conducted at the JINR U400 cyclotron between February and June 2005, the researchers observed atomic decay patterns, or chains, that establish the existence of element 118. In these decay chains, previously observed element 116 is produced via the alpha decay of element 118.

The results will be published in the October 2006 edition of the journal, Physical Review C.
The experiment produced three atoms of element 118 when calcium ions bombarded a californium target. The team then observed the alpha decay from element 118 to element 116 and then to element 114. The Livermore-Dubna team had created the same isotope of element 116 in earlier experiments.
This discovery brings the total to five new elements for the Livermore-Dubna collaboration (113, 114, 115, 116 and 118).
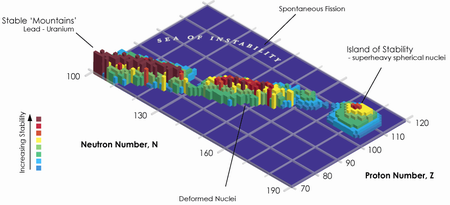
"The decay properties of all the isotopes that we have made so far paint the picture of a large, sort of flat Island of Stability and indicate that we may have luck if we try to go even heavier," said Ken Moody, Livermore's team leader.
The Island of Stability is a term from nuclear physics that describes the possibility of elements, which have particularly stable "magic numbers" of protons and neutrons. This would allow certain isotopes of some transuranic elements (elements with atomic numbers greater than 92) to be far more stable than others, and thus decay much more slowly. Element 118 is expected to be a noble gas that lies right below radon on the periodic table of elements. The world is made up of about 90 elements," Moody said. "Anything more you can learn about the periodic table is exciting. It can tell us why the world is here and what it is made of."
"This is quite a breakthrough for science," said Chemistry, Materials and Life Sciences Associate Director Tomas Diaz de la Rubia. "We've discovered a new element that provides insight into the makeup of the universe. For our scientists to find another piece of the puzzle is a testament to the strength and value of the science and technology at this Laboratory."
Livermore has had a long-standing heavy element group since the inception of the Laboratory in 1952. The group has been successful in the discovery of several new elements over the years because it has access to unique materials to perform the experiments. In 1999 and 2001, the Laboratory announced the discovery of elements 114 and 116, respectively. In 2004, the Livermore-Dubna team observed the existence of elements 113 and 115.
As for the future, the LLNL-Dubna team will continue to map the region near the "Island of Stability." In 2007, the team plans to look for element 120 by bombarding a plutonium target with iron isotopes.
"The heavy element community will continue to search for new elements until the limit of nuclear stability is found," Mark Stoyer said. "It is expected that limit will be found."
Members of the Livermore team include: Moody, Dawn Shaughnessy, Mark Stoyer, Nancy Stoyer, Philip Wilk, Jacqueline Kenneally, Jerry Landrum, John Wild, Ron Lougheed and former LLNL employee Joshua Patin.
Citation: The paper appears online at link.aps.org/abstract/PRC/v74/e044602
Source: Lawrence Livermore National Laboratory, American Physical Society