Organic semiconductors make cheap, flexible photovoltaics and LEDs
Imagine T-shirts that light up, or a beach umbrella that collects solar energy to run a portable TV. How about really cheap solar collectors for the roof? All this and more could come from cutting-edge research at Cornell that demonstrates a new type of organic semiconductor device which shows electroluminescence and acts as a photovoltaic cell.
The device is the first to use an "ionic junction," which researchers say could lead to improved performance. Since organic semiconductors can be made in thin, flexible sheets, they could create displays on cloth or paper.
"Flexible means low-cost fabrication," said George Malliaras, Cornell associate professor of materials science and engineering, in whose laboratory the research was done. And that means another result of the research could be mass-produced, inexpensive solar cells.
The work is described in the Sept. 7 issue of the journal Science in a paper by Cornell graduate researchers Daniel Bernards and Samuel Flores-Torres, Héctor Abruña, the E. M. Chamot Professor of Chemistry and Chemical Biology at Cornell, and Malliaras.
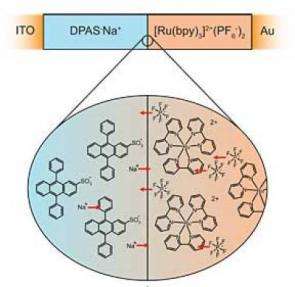
Semiconductors -- organic or otherwise -- are materials that contain either an excess of free electrons (N-type) or "holes" (P-type). Holes are spaces where an atom ought to have an electron but doesn't, representing a positive charge. N- and P-type materials can be joined to form diodes and transistors. The Cornell researchers went a step further by making a diode out of organic semiconductors that also contain free ions (molecules with an electrical charge). They laminated together two organic layers, one that contained free positive ions and the other negative ions. They then added thin conducting films on the top and bottom; the top conductor is transparent to allow light in and out.
Where the two films meet, negative ions migrate across the junction to the positive side and vice versa, until an equilibrium is reached. This is analogous, the researchers said, to what happens in a silicon diode, where electrons and holes migrate across the junction.
When a voltage is applied across the top and bottom electrodes, a current flows through the junction in the form of electrons moving one way while holes move the other way. The migration of ionic charge across the junction causes a higher potential (voltage difference) than normal, which affects the way electrons combine with holes. This raises the energy of the molecules, which quickly release the energy as photons of light. The junction shows "intense light emission," the researchers said in their paper.
On the other hand, when a bright light is applied, photons are absorbed by the molecules, causing them to kick out electrons. The ionic charges create a "preferential direction" for the electrons to move, and a current flows.
The collection of charges also allows electrons and holes to move across the junction easily in one direction but only weakly in the other, making the device a rectifier. It may be possible, Malliaras said, to change the configuration of the ionic charge by applying a voltage to the device, telling it whether to conduct or not, so organic diodes might be used as components for computer memory.
Since the device was created by laminating together materials that are flexible, large quantities could be manufactured very cheaply by feeding two films together from rolls, Malliaras said. The next step, he added, is to try modifying the metal content of the semiconductors to make more efficient materials.
"There are tons of materials we can use," he said.
Source: Cornell University