Blood clot fibers more elastic than spider's web
The tiny fibers that comprise blood clots show extraordinary elasticity, on average stretching to almost three times their length while still retaining their ability to go back to their normal shape and expanding to more than four times their length before breaking, according to findings published in the journal Science this week by researchers at Wake Forest University.
This discovery, which makes these fibrin fibers the most stretchable known fibers existing in nature, will help medical researchers create more accurate blood clot models, provide new insights into the wound healing process and offer a deeper understanding of heart attacks and strokes. Researchers from Wake Forest's physics department and Wake Forest University School of Medicine worked closely with researchers from the University of North Carolina at Chapel Hill on this project.
"For all naturally occurring fibers, fibrin fibers are the ones you can stretch the furthest before they break," said Martin Guthold, assistant professor of physics and one of the lead authors of the paper that appears in Science. "This was a stunning revelation because people hypothesized that these fibers stretched but broke much easier. In some cases, fibrin fibers had the ability to be stretched more than six times their length before they broke."
Blood clots are a three-dimensional network or mesh of fibrin fibers, stabilized by another protein called factor XIIIa. Because of its important function of stemming the flow of blood in the body, clots have to be both strong and pliable.
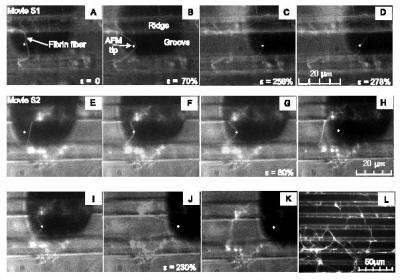
Fibrin fibers measure about 100 nanometers in diameter, roughly 1,000 times smaller than a human hair.
"The fibrin fibers need to stop the flow of blood, so there is a lot of mechanical stress on those fibers," Guthold said. "Our discovery of these mechanical properties of individual fibrin fibers shows that these fibers likely endow blood clots with important physiological properties. They make blood clots very elastic and very stretchable."
Scientists had previously been unable to study the mechanical properties of individual fibrin fibers because of their small size.
Guthold and his research team at Wake Forest created a device by combining two microscopes that could not only see the fibrin fibers but also stretch the fibers. The fibers were suspended across a channel and anchored to the ridges of the channel at right angles. The fibers were stretched using the tip of an atomic force microscope. Movies showing the stretching are available at www.wfu.edu/%7Egutholdm/research.html
Results showed that on average fibrin fibers can be stretched to 4.3 times their length before breaking. In addition, the fibers can be stretched to more than 2.8 times their length and still recover without permanent damage.
Roy Hantgan, associate professor of biochemistry at Wake Forest University School of Medicine and a member of the research team, said the study findings have significant implications for human health.
"Knowing that the fibrin strands that make up a human blood clot are more stretchable than a spider's web helps us to understand how clots can seal wounds tightly and withstand the pressure in our blood vessels," Hantgan said. "This new information also helps us to understand how tough it is to remove a clot that is preventing blood flow to a person's heart or brain, causing a heart attack or stroke."
Guthold said he has already been contacted by a company that uses an ultrasonic device to break up blood clots. He said the company has an interest in knowing the properties of the fibers comprising a blood clot to determine how much force should be applied to break up clots.
Researchers from Guthold's lab who worked on the project include Wenhua Liu, a physics graduate student at Wake Forest; and Eric Sparks, an undergraduate student at Wake Forest. Susan Lord, professor of pathology and medicine at UNC-Chapel Hill, is a lead researcher on the study, along with Guthold.
Source: Wake Forest University





















