Scientists observe the transition state in a chemical reaction on a catalyst's surface
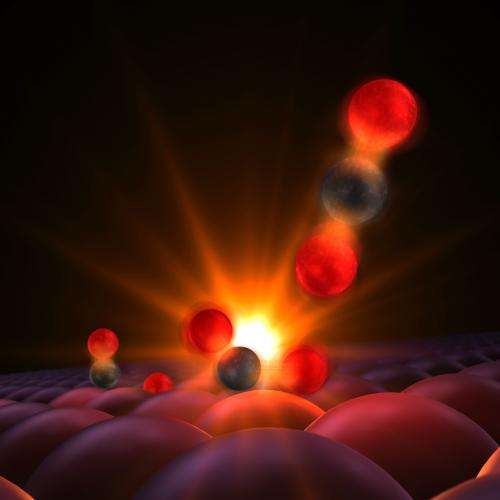
For the first time, an international scientists' team observed the volatile intermediate stages which appear when carbon monoxide oxidises on a hot ruthenium surface, an ordinary catalyst. The scientists, including researchers from the University of Hamburg and DESY, used the ultra-short X-ray flashes of the X-ray laser LCLS at the research centre SLAC in California. Initially, an optical laser pulse heated the ruthenium surface, thus activating the absorbed carbon monoxide molecules and oxygen atoms. With the help of X-ray absorption spectroscopy, the team was able to detect how the electronic structure of the involved oxygen atoms changed during the formation of carbon dioxide molecules – a process which regularly takes place in a similar way in all automobile catalysts. The observed transition states assort well with quantum chemical calculations.
Surprising, however, was the observation of how many reaction partners were activated in a transition state, and equally surprising was the discovery that only a small fraction of them actually formed stable CO2 molecules. "It is like shooting marbles up a hill, and most of them which reach the top just roll down again at the same side of the hill," said Anders Nilsson, Professor at SLAC/Stanford SUNCAT Center for Interface Science and Catalysis and at Stockholm University, who headed the research project.
Free-electron lasers allow time resolutions of less than 100 femtoseconds which are required for the direct observation of such transition states in chemical reactions. These investigations are a central research theme in the Hamburg Cluster of Excellence "Centre for Ultrafast Imaging" (CUI), with the University of Hamburg, European XFEL, EMBL and DESY as partners.
The group of Wilfried Wurth at the Center for Free-Electron Laser Science (CFEL) participates in this project within the framework of their CUI activities. Wilfried Wurth, who is also scientific head of FLASH, considers the experiments at LCLS, which at the same time are a benchmark for the quantum chemical calculations, to be "a first step on the road to a 'molecular movie', which allows watching a catalyst working under realistic conditions." According to Wurth, experiments of this kind, which do not only observe the molecules but also include the dynamic changes of the active catalyst's surface, will be possible in the future with the European X-ray laser facility European XFEL, currently under construction in the Hamburg area.
More information: "Probing the transition state region in catalytic co oxidation on RU." Science 1261747 DOI: 10.1126/science.1261747
Journal information: Science
Provided by Deutsches Elektronen-Synchrotron