February 13, 2015 report
Researchers gain better understanding of cellular intestinal barrier structure
(Phys.org)—A team of researches affiliated with several institutions in Japan has conducted research into the cellular structure of tight junctions in the small intestine, and has made progress in better understanding their construction, possibly helping to pave the way towards creating drugs that would be better equipped to make their way into the bloodstream. In their paper published in the journal Science, the team describes their research and findings and what they believe must be done next. Per Artursson and Stefan Knight with Uppsala University in Sweden offer a perspective piece on the work done by the team in the same journal edition.
In order for drugs taken orally to do their job, they must make their way through the stomach, and into the small intestine. Once there, they must pass through a layer of endothelial cells that make up a protective barrier meant to keep out harmful substances. Unlike the blood-brain barrier that exists in capillaries that are impermeable, the barrier in the small intestine is semi-permeable, allowing some substances to pass through, but not others. Those that do get through, find their way through what are known as tight junctions, the spaces between cells.
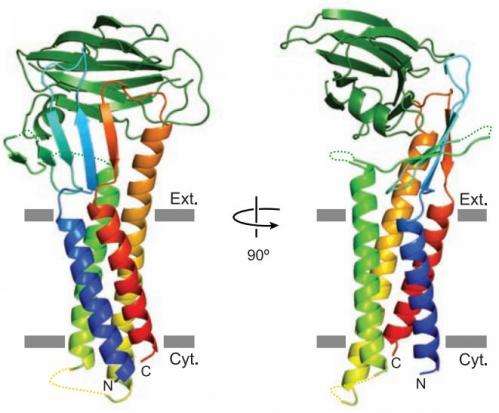
In this new effort the researchers learned more about the crystal structure of claudin-19, one of the major proteins that exist in the barrier, by watching how it was impacted by a bacterial toxin known as CPE—prior research has shown that it breaks down material in the tight junctions allowing entry into the bloodstream and causing food borne illnesses.
This new research was focused on learning more about how CPE causes destruction of claudin-19—the researchers found that it came about due to the bacteria's ability to block the formation of a short, extracellular helix, which serves as a physical barrier. Without the barrier, CPE can pass right through. As Artursson and Knight point out, the new research did not lead to a clear understanding or explanation of just how CPE was able to block the formation of the helix, but it does offer a new path to take. If researchers can figure out just how exactly CPE blocks formation of the helix, the process might be duplicated in substances added to medicines, allowing future patients to take medicine orally that must now be given by injection.
More information: Structural insight into tight junction disassembly by Clostridium perfringens enterotoxin, Science 13 February 2015: Vol. 347 no. 6223 pp. 775-778. DOI: 10.1126/science.1261833
ABSTRACT
The C-terminal region of Clostridium perfringens enterotoxin (C-CPE) can bind to specific claudins, resulting in the disintegration of tight junctions (TJs) and an increase in the paracellular permeability across epithelial cell sheets. Here we present the structure of mammalian claudin-19 in complex with C-CPE at 3.7 Å resolution. The structure shows that C-CPE forms extensive hydrophobic and hydrophilic interactions with the two extracellular segments of claudin-19. The claudin-19/C-CPE complex shows no density of a short extracellular helix that is critical for claudins to assemble into TJ strands. The helix displacement may thus underlie C-CPE–mediated disassembly of TJs.
PERSPECTIVE: Breaking the intestinal barrier to deliver drugs, Science, www.sciencemag.org/content/347/6223/716.summary
Journal information: Science
© 2015 Phys.org










.jpg)