Drastic reduction in the use of synthetic surfactants with power from microorganisms
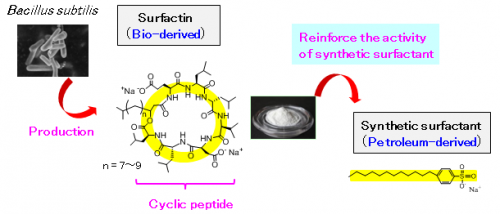
Researchers at the National Institute of Advanced Industrial Science and Technology, in collaboration with Kaneka Corporation, have discovered that a cyclic peptide (surfactin) produced from Bacillus subtilis (B. subtilis) can reduce the necessary amount of synthetic surfactants to approximately one hundredth.
With the growing awareness towards a low-carbon society, there is a need to reduce the use of synthetic surfactants where concerns exist for their dispersal into the environment and the replacement of petroleum-derived surfactants with bio-derived ones is being required. A detailed study of the characteristics of surfactin, which can be efficiently produced using B. subtilis, has revealed that the action of the bulky cyclic peptide structure significantly increases the activity of synthetic surfactants. It has been proven that, by adding a small amount of surfactin to a petroleum-derived surfactant, equal or higher surfactant effects can be maintained even when the amount of synthetic surfactant has been reduced to one hundredth. The utilization of the discovered effects is expected to significantly decrease the amount of synthetic surfactants used widely in daily goods such as detergents and shampoos as well as in a wide range of chemical products.
The details of this technology will be presented at the 53rd Annual Meeting of the Japan Oil Chemists' Society to be held on September 9 - 11, 2014 in Sapporo, Hokkaido.
Synthetic surfactants, together with plastics, are the representative petroleum based products and approximately 1 million tons are manufactured in Japan alone. They are used widely in daily products such as dish detergents and shampoos as well as in machinery, construction, civil engineering and other industries. Since there is a possibility that synthetic surfactants will be dispersed into the environment after use, development of high-functional new products that can show their function by a small amount and new technology to reduce the use of surfactants are now becoming important.
Furthermore, with environmental issues such as global warming becoming apparent, conversion from petroleum-derived to bio-derived chemical products is being required, with the aim of moving away from fossil resources. While cyclic peptides have displayed various functions, including antibacterial characteristics, applications mainly in the pharmaceutical area had been studied due to their high production cost. Recently, they are becoming to be known to have excellent surfactant effects (detergent action, etc.) and there are expectations for application in areas other than pharmaceuticals, as well as the establishment of mass production technology.
AIST has been conducting research into bio-derived chemical products as a part of its eco-friendly chemicals development (AIST press release on August 29, 2013), and has been consistently conducting the development of production technologies using microorganism fermentation processes, and application research into their performance evaluation, application development, etc.
Using its unique fermentation and refinement technologies, Kaneka has succeeded in the mass production of surfactin, which previously had problems in mass production for various reasons, such as its low productivity in its cultivation process, and was looking for new applications in various industries. In order to expedite application deployment, it was necessary to conduct fundamental R&D focusing on its unique cyclic peptide structure, and joint research was started with AIST.
Surfactin, that Kanaka had successfully mass produced, is a peptide in which amino acids are bonded in a cyclic form. It places a low burden on the environment and living organisms. As it is very different from synthetic surfactants (linear form), it has been expected to show unique functions.
AIST began its research on surfactin by assessing its assembly characteristics in aqueous solution. In general, surfactant molecules line up with regularity on the water surface and when the surface is saturated, the surfactant molecules move into the solution and form assemblies called micelle that display detergency. Therefore, the lower the micelle formation concentration (critical micelle concentration) is, the lower the necessary amount of the surfactant.
In the present research, in addition to its molecular size being 3 to 5 times larger than a synthetic surfactant, surfactin has been found to line up on the water surface more easily. This being the case, it was presumed that when surfactin and a synthetic surfactant are used together, the water surface would first be covered and saturated with surfactin, promoting micelle formation, and would significantly decrease the micelle formation concentration of the synthetic surfactant.
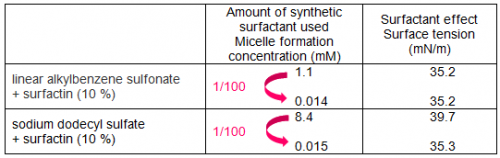
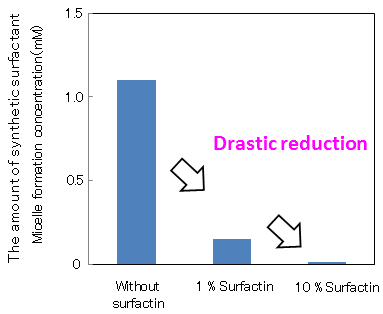
Figure 1 shows the surfactant effects, when a small amount of surfactin was added to linear alkylbenzene sulfonate, a major component of detergents. By adding 1 % surfactin to the synthetic surfactant, micelle formation concentration fell to one tenth and even when the amount of synthetic surfactant was reduced to one tenth of its original concentration, equal or larger surfactant effects (surface tension-lowering ability) were maintained. Furthermore, when 10 % surfactin was added to the synthetic surfactant, equal or larger surfactant effects were displayed even when the amount of synthetic surfactant was reduced to one hundredth.
In addition, the effects of surfactin addition to sodium dodecyl sulfate, another type of petroleum-derived synthetic surfactant, were investigated (Table 1). As a result, when 10 % surfactin was added to sodium dodecyl sulfate, the micelle formation concentration fell to one hundredth and it was found that even if the concentration of the synthetic surfactant was lowered to about one hundredth, equivalent or larger surfactant effects could be maintained.
Applying the effects of surfactin addition to daily products such as detergents and shampoos and to a wide range of chemical products is expected to significantly decrease the quantity of synthetic surfactants in the products.
Further assessment of the functions of surfactin will be conducted to promote the use of surfactin in various areas. In addition, discovery and development shall be continued for amino acids and peptide materials with new structures and characteristics, to contribute to the transition from petroleum-derived to bio-derived chemical products and the promotion of manufacturing and use of bio-derived chemical products.
Provided by Advanced Industrial Science and Technology

















