Transition metal catalysts could be key to origin of life, scientists report
One of the big, unsolved problems in explaining how life arose on Earth is a chicken-and-egg paradox: How could the basic biochemicals -- such as amino acids and nucleotides -- have arisen before the biological catalysts (proteins or ribozymes) existed to carry out their formation?
In a paper appearing in the current issue of The Biological Bulletin, scientists propose that a third type of catalyst could have jumpstarted metabolism and life itself, deep in hydrothermal ocean vents.
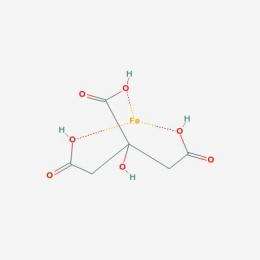
According to the scientists' model, which is experimentally testable, molecular structures involving transition metal elements (iron, copper, nickel, etc.) and ligands (small organic molecules) could have catalyzed the synthesis of basic biochemicals (monomers) that acted as building blocks for more complex molecules, leading ultimately to the origin of life. The model has been put forth by Harold Morowitz of George Mason University (GMU), Vijayasarathy Srinivasan of GMU, and Eric Smith of the Santa Fe Institute.
"There has been a big problem in the origin of life (theory) for the last 50 years in that you need large protein molecules to be catalysts to make monomers, but you need monomers to make the catalysts," Morowitz says. However, he suggests, "You can start out with these small metal-ligand catalysts, and they'll build up the monomers that can be used to make the (large protein catalysts)."
A transition metal atom can act as the core of a metal-ligand complex, in which it is bound to and surrounded by other ligands. Morowitz and his colleagues propose that simple transition metal-ligand complexes in hydrothermal ocean vents catalyzed reactions that gave rise to more complex molecules. These increasingly complex molecules then acted as ligands in increasingly efficient transition metal-ligand complex catalysts. Gradually, the basic molecular ingredients of metabolism accumulated and were able to self-organize into networks of chemical reactions that laid the foundation for life.
"We used to think if we could understand what carbon, hydrogen, nitrogen, oxygen, phosphorus, and sulfur were doing, we would immediately be able to understand biology," Morowitz says, listing elements that constitute a large proportion of Earth's biomass. "But now we're finding that these other fairly rare elements, transition metals, are necessary in biology, so we ask, 'What was their role in the origin of life?'"
The proposal suggests that the rise of life forms is a natural consequence of the unique properties of transition metals and ligand field theory, which describes the characteristics of ligand complexes.
"The idea has emerged from a study of the periodic table. We strongly feel that unless you're able to see how life comes about in some formal chemical way, you're never really going to solve the problem," Morowitz says.
Morowitz and his colleagues are preparing experiments to test the catalytic properties of transition metal-ligand complexes built with different types of ligands. Ligands known to bind tightly to transition metals include molecules produced during the course of the reductive citric acid cycle, a series of biochemical reactions essential for many microorganisms.
"We think life probably began with the reductive citric acid cycle, and there is evidence that under hydrothermal vent conditions some of the cycle's intermediates form," Morowitz says. "We are going to start with these molecules and mix them with various transition metals, cook them at different temperatures for a while, and see what kinds of catalysts we've made."
Such experiments could reveal what kinds of catalytic reactions took place to lay the foundations for life. The hypothesis also allows for the possibility that life could have arisen more than once.
"Life could have originated multiples times, and, if we find life elsewhere in the universe, it could be very similar to the life we know here because it will be based on the same transition metals and ligands," Morowitz says. "It's a conjecture at the moment, but it could become a formal scientific core for the emergence of life."
More information: Morowitz, H. J., Srinivasan, V., Smith, E. (2010) Ligand Field Theory and the Origin of Life as an Emergent Feature of the Periodic Table of Elements. Biol. Bull. 219: 1-6.
Provided by Marine Biological Laboratory