A simple quantum dynamics problem?
Research reported in The Journal of Chemical Physics, which is published by the American Institute of Physics, provides the first real-time measurements of the time dependence of the individual steps of dissociation of a complex consisting of two rare gas atoms and a halogen molecule.
"The goal of this work is to provide a test case for quantum dynamics theory," says author Kenneth C. Janda of the University of California, Irvine. "It is a problem that is easy, but not too easy, in the sense that a fundamental quantum dynamics explanation is within reach."
Researchers cooled a mixture of helium, neon, and bromine by spraying it through a nozzle, resulting in a stream of gas particles traveling at the same speed. This created a very low temperature in a moving frame of reference -- the particles were stationary relative to one another and condensed to form Ne2Br2 tetrahedral complexes. After the bromine molecule was excited with a laser pulse, the dissociation of the complex over a period of tens of picoseconds was observed spectroscopically.
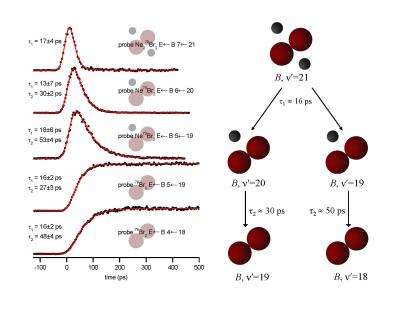
Adding 16 quanta of vibrational energy to the bromine-stretching vibration resulted in rapid direct dissociation. The two Ne atoms dissociated without interacting with each other. However, with slightly higher vibrational excitation, a 23-quanta boost, the bromine anharmonicity led to sharing of the kinetic energy between the Ne atoms and a much more complicated dissociation mechanism.
"For 23 quanta, the first transfer of vibration fails to knock off one of the neon atoms 80 percent of the time," says Janda. "Instead a tiny liquid drop is formed, allowing a neon atom to move in a direct line with the bromine atoms. The next vibration shoots it off like a pool stick hitting the cue ball."
More information: The article "Real-time dissociation dynamics of the Ne2Br2 van der Waals complex" by Jordan M. Pio, Molly A. Taylor, Wytze E. van der Veer, Craig R. Bieler, Jose A. Cabrera, and Kenneth C. Janda was published online in The Journal of Chemical Physics on July 7, 2010. See: link.aip.org/link/JCPSA6/v133/i1/p014305/s1
Provided by American Institute of Physics