Solar cells: UQAM researcher solves two 20-year-old problems
Thanks to two technologies developed by Professor Benoit Marsan and his team at the Universite du Quebec a Montreal (UQAM) Chemistry Department, the scientific and commercial future of solar cells could be totally transformed. Professor Marsan has come up with solutions for two problems that, for the last twenty years, have been hampering the development of efficient and affordable solar cells.
His findings have been published in two prestigious scientific journals, the Journal of the American Chemical Society (JACS) and Nature Chemistry.
The untapped potential of solar energy
The Earth receives more solar energy in one hour than the entire planet currently consumes in a year! Unfortunately, despite this enormous potential, solar energy is barely exploited. The electricity produced by conventional solar cells, composed of semiconductor materials like silicon, is 5 or 6 times more expensive than from traditional energy sources, such as fossil fuels or hydropower. Over the years, numerous research teams have attempted to develop a solar cell that would be both efficient in terms of energy and inexpensive to produce.
Dye-sensitized solar cells
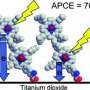
One of the most promising solar cells was designed in the early '90s by Professor Michael Graetzel of the Ecole Polytechnique Federale de Lausanne (EPFL) in Switzerland. Based on the principle of photosynthesis—the biochemical process by which plants convert light energy into carbohydrate (sugar, their food)—the Graetzel solar cell is composed of a porous layer of nanoparticles of a white pigment, titanium dioxide, covered with a molecular dye that absorbs sunlight, like the chlorophyll in green leaves. The pigment-coated titanium dioxide is immersed in an electrolyte solution, and a platinum-based catalyst completes the package.
As in a conventional electrochemical cell (such as an alkaline battery), two electrodes (the titanium dioxide anode and the platinum cathode in the Graetzel cell) are placed on either side of a liquid conductor (the electrolyte). Sunlight passes through the cathode and the electrolyte, and then withdraws electrons from the titanium dioxide anode, a semiconductor at the bottom of the cell. These electrons travel through a wire from the anode to the cathode, creating an electrical current. In this way, energy from the sun is converted into electricity.
Most of the materials used to make this cell are low-cost, easy to manufacture and flexible, allowing them to be integrated into a wide variety of objects and materials. In theory, the Graetzel solar cell has tremendous possibilities. Unfortunately, despite the excellence of the concept, this type of cell has two major problems that have prevented its large-scale commercialisation:
- The electrolyte is: a) extremely corrosive, resulting in a lack of durability; b) densely coloured, preventing the efficient passage of light; and c) limits the device photovoltage to 0.7 volts.
- The cathode is covered with platinum, a material that is expensive, non-transparent and rare.
Professor Marsan's solutions
Professor Marsan and his team have been working for several years on the design of an electrochemical solar cell. His work has involved novel technologies, for which he has received numerous patents. In considering the problems of the cell developed by his Swiss colleague, Professor Marsan realized that two of the technologies developed for the electrochemical cell could also be applied to the Graetzel solar cell, specifically:
- For the electrolyte, entirely new molecules have been created in the laboratory whose concentration has been increased through the contribution of Professor Livain Breau, also of the Chemistry Department. The resulting liquid or gel is transparent and non-corrosive and can increase the photovoltage, thus improving the cell's output and stability.
- For the cathode, the platinum can be replaced by cobalt sulphide, which is far less expensive. It is also more efficient, more stable and easier to produce in the laboratory.
More information:
Links to the articles in JACS and Nature Chemistry:
pubs.acs.org/doi/abs/10.1021/ja905970y
www.nature.com/nchem/journal/v … t/abs/nchem.610.html
Provided by Universite du Quebec a Montreal