Accidental discovery produces durable new blue pigment for multiple applications

An accidental discovery in a laboratory at Oregon State University has apparently solved a quest that over thousands of years has absorbed the energies of ancient Egyptians, the Han dynasty in China, Mayan cultures and more - the creation of a near-perfect blue pigment.
Through much of recorded human history, people around the world have sought inorganic compounds that could be used to paint things blue, often with limited success. Most had environmental or durability issues. Cobalt blue, developed in France in the early 1800s, can be carcinogenic. Prussian blue can release cyanide. Other blue pigments are not stable when exposed to heat or acidic conditions.
But chemists at OSU have discovered new compounds based on manganese that should address all of those concerns. They are safer to produce, much more durable, and should lead to more environmentally benign blue pigments than any being used now or in the past. They can survive at extraordinarily high temperatures and don't fade after a week in an acid bath.
The findings were just published in the Journal of the American Chemical Society, and a patent has been applied for on the composition of the compound and the process used to create it. The research was funded by the National Science Foundation.
"Basically, this was an accidental discovery," said Mas Subramanian, the Milton Harris Professor of Materials Science in the OSU Department of Chemistry. "We were exploring manganese oxides for some interesting electronic properties they have, something that can be both ferroelectric and ferromagnetic at the same time. Our work had nothing to do with looking for a pigment.
"Then one day a graduate student who is working in the project was taking samples out of a very hot furnace while I was walking by, and it was blue, a very beautiful blue," he said. "I realized immediately that something amazing had happened."
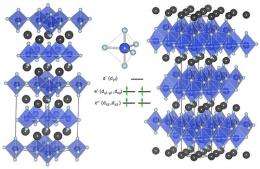
What had happened, the researchers said, was that at about 1,200 degrees centigrade - almost 2,000 degrees Fahrenheit - this otherwise innocuous manganese oxide turned into a vivid blue compound that could be used to make a pigment able to resist heat and acid, be environmentally benign and cheap to produce from a readily available mineral.
The newest - and possibly the best - blue pigment in world history was born, due to manganese ions being structured in an unusual "trigonal bipyramidal coordination" in the presence of extreme heat.
"Ever since the early Egyptians developed some of the first blue pigments, the pigment industry has been struggling to address problems with safety, toxicity and durability," Subramanian said.
The pigment may eventually find uses in everything from inkjet printers to automobiles, fine art or house paint, researchers say.
The scientists said in their journal article that the new compound yields "a surprisingly intense and bright blue color," and they have outlined its structure and characteristics in detail. Collaborating on the work were researchers in the Materials Department at the University of California/Santa Barbara.
"A lot of the most interesting discoveries are not really planned, we've seen that throughout history," Subramanian said. "There is luck involved, but I also teach my students that you have to stay alert to recognize something when it happens, even if it isn't what you were looking for."
"Luck favors the alert mind."
Source: Oregon State University (news : web)

















