August 28, 2009 weblog
Scientists Image the 'Anatomy' of a Molecule (w/ Video)
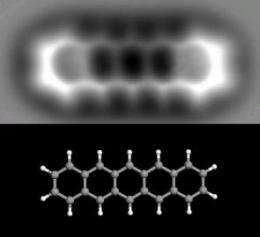
(PhysOrg.com) -- For the first time, IBM researchers in Zurich, Switzerland, have taken a 3D image of an individual molecule. Using an atomic force microscope, the researchers constructed a "force map" of pentacene, an organic molecule just 1.4 nanometers long. As the researchers explain, the technique is roughly analogous to how an x-ray machine images bones in the human body by looking through flesh. In this case, the scientists could look through the electron cloud and see the atomic backbone of the molecule.
To create an image, the atomic force microscope uses a sharp metal tip to measure the tiny forces between the tip and the pentacene molecule. Pentacene is an oblong molecule that consists of 22 carbon atoms and 14 hydrogen atoms, with the carbon atoms spaced just 0.14 nanometers apart. In the image, the five hexagonal shaped carbon rings, the carbon atoms, and the positions of the hydrogen atoms can be seen.
Although researchers have previously imaged atoms, imaging molecules is more difficult due to their fragility. While techniques such as transmission electron microscopy can bombard materials with electrons in order to view atoms, the electron bombardment destroys the arrangement of atoms in molecules.
To overcome these challenges, the IBM researchers, led by Leo Gross, modified the atomic force microscope technique. The team used a tip with a carbon monoxide molecule, held just 0.4 mm above the molecule, to balance competing forces that occur at this tiny range. While the attractive van der Waals force tries to pull the microscope tip and molecule together, a quantum mechanical effect based on the Pauli exclusion principle repels the electrons around the pentacene and those around the carbon monoxide molecule.
By measuring the repulsive force of the tip at each point, the researchers could construct the force map of the molecule. To achieve significant detail, the researchers focused the microscope for 20 hours of data acquisition, operating in an ultrahigh vacuum at very low temperatures (5 Kelvin).
As IBM stated in a press release, the results push the exploration of using molecules and atoms at the smallest scale and could greatly impact the field of nanotechnology.
"Scanning probe techniques offer amazing potential for prototyping complex functional structures and for tailoring and studying their electronic and chemical properties on the atomic scale," said IBM researcher Gerhard Meyer.
For instance, the technique could open the door to more powerful computers whose components are built with precisely positioned atoms and molecules. Researchers could also gain insight into molecular-level activity, such as the actions of catalysts in reactions, and how molecular geometry changes when changing the charge of a molecule.
"These breakthroughs will open new possibilities for investigating how charge transmits through molecules or molecular networks," IBM stated. "Understanding the charge distribution at the atomic scale is essential for building smaller, faster and more energy-efficient computing components than today's processors and memory devices."
More information:
Leo Gross, Fabian Mohn, Nikolaj Moll, Peter Liljeroth, and Gerhard Meyer. "The Chemical Structure of a Molecule Resolved by Atomic Force Microscopy." Science, 28 August 2009: Vol. 325. no. 5944, pp. 1110 - 1114. DOI: 10.1126/science.1176210.
© 2009 PhysOrg.com



















