Revealing the long-awaited atomic structure of a well-known enzyme
A Boston University-led research team has identified the structural underpinnings of a widely-known enzyme -- acetoacetate decarboxylase (AADase) -- that was first described correctly more than 43 years ago including how it accelerates its target reaction. Until now it has never been fully explained how the reactions occur in the environment of the cell.
Enzymes catalyze, or speed-up, chemical processes by accomplishing in seconds what otherwise might take hours, days or centuries to happen spontaneously. AADase is the catalyst that that converts acetoacetate to acetone, a key component in the metabolism of carbohydrates in bacteria and a historically important enzyme whose industrial development - the conversion of acetoacetate to acetone -- for cordite, the colorless, flammable substance used during World War 1 to make explosives for naval guns and as a coating for military airplane wings.
Back then Chaim Weiszmann, a Russian chemist living in England isolated a bacterium that transforms cornstarch to a mixture of acetone, a common building block in organic chemistry, and several alcohols - a major improvement over an inefficient wood distillation process in use at that time. The manufacturing process, which was developed and scaled up with U.S. and Canadian help, ultimately enabled the British in 1917 to win the war over Germany.
In the 1960s, AADase was used by Dr. Frank H. Westheimer, a Harvard University chemistry professor, to pioneer the application of methods in physical organic chemistry for the study of the chemical and catalytic mechanism of enzymes. He developed a hypothesis about how enzymes worked, and how a protein could control its own environment around the reaction. By changing the interior of a protein, chemical reactions occurred that were different from those that can occur in solution and from those that occur in other enzymes in the cell.
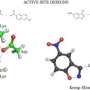
Missing from Westheimer's model was a structural explanation that would show the basis for his electrostatic perturbation hypothesis. Although the hypothesis has been demonstrated in a number of enzymes, there were assumptions made about the complex three-dimensional shape -- or protein fold which provides AADase's structure.
By defining AADase atomic structure using X-ray crystallography, the research team corrects those assumptions and provides the missing structure that explains Westheimer's hypothesis about microenvironmental control of enzyme reactions within the cell.
The research, entitled "The origin of the electrostatic perturbation in acetoacetate decarboxylase," appears online today in Nature. Its authors are Boston University chemistry professor Karen N. Allen, Ph.D., BU graduate student Meng-Chiao Ho and post-doctoral associate Jean-Francois Menetret, and Hiro Tsuruta of the Stanford Synchrotron Radiation Lightsource of the National Accelerator Laboratory.
"Westheimer was right about the enzyme controlling the microenvironment around the reaction, but the way it did it was completely different than he and his research team supposed," said Allen, who named the protein fold she discovered after Westheimer .
Proteins are the biological workhorses that provide the vital functions in every cell. To carry out their tasks, proteins must fold into the complex three dimensional shapes that provide their structure.
"That's the importance of this finding, she added. "Now that we know the structure, we can go back and correct all the text books citing the origin of electrostatic effects in enzyme active sites and say that while the hypothesis was right - and proven for other enzymes -- the basis for it was actually different than what they originally believed."
With a structure fully identified, researchers hope to gain new insights into predicting the functions of other enzymes. The discovery of novel structures also allows researchers to find the active sites and therefore the chemical roles of enzymes of unknown function, uncovering new metabolic pathways in the cell.
An understanding of the molecular detail of an enzyme, like AADase may also enable researchers in protein engineering to alter its structure to work with specific solvents to develop new environmentally friendly "green" biofuels.
Source: Boston University Medical Center