Turn back, wayward axon
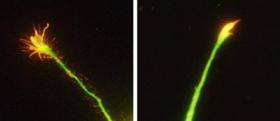
To a growing axon, the protein RGMa is a "Wrong Way" sign, alerting it to head in another direction. As Hata et al. demonstrate in the March 9, 2009 issue of the Journal of Cell Biology, translating that signal into cellular action requires teamwork from two receptors.
During development, new synapses form when the growing axon of one neuron reaches another neuron. As an axon searches out the path to its destination, it bends toward so-called attractive guidance molecules and veers away from repulsive guidance molecules such as RGMa. For example, if the tip of an axon touches a glial cell instead of a neuron, the extension pulls back. On its membrane the glial cell sports RGMa, which latches onto the receptor neogenin on the axon. Researchers knew that the interaction between RGMa and neogenin halted the axon by activating the GTPase RhoA. However, they didn't know how neogenin switches on RhoA.
Hata et al. discovered that it gets help from another axon membrane receptor called Unc5B. The researchers found that after a dose of RGMa, the tip of a growing axon halted and often retreated. Eliminating Unc5B prevented this collapse.
Neogenin and Unc5B stick together and serve as coreceptors, performing slightly different tasks, Hata et al. conclude. Neogenin's job is to hook up with RGMa. Unc5B, by contrast, never contacts RGMa. Instead, it serves as a docking point for the RhoA activator LARG. Unc5B indirectly switches on RhoA by interacting with LARG.
But that left one further mystery to explain. LARG clings to Unc5B all the time, so why does it fire up RhoA only in response to RGMa? The researchers found that binding of RGMa prodded another protein, the focal adhesion kinase (FAK), to switch on LARG, allowing activation of RhoA. How RGMa binding triggers FAK is the next question the researchers want to answer.
More information: www.jcb.org, Hata, K., et al. 2009. J. Cell Biol. doi:10.1083/jcb.200807029.
Source: Rockefeller University