Single molecule tracking helps reveal mechanism of chromosome separation in dividing cells

(PhysOrg.com) -- University of Washington (UW) researchers are helping to write the operating manual for the nano-scale machine that separates chromosomes before cell division. The apparatus is called a spindle because it looks like a tiny wool-spinner with thin strands of microtubules or spindle fibers sticking out. The lengthening and shortening of microtubules is thought to help push and pull apart chromosome pairs.
Understanding how this machine accurately and evenly divides genetic material is essential to learning why its parts sometimes fail. Certain cancers or birth defects, like Down syndrome or Trisomy 18, result from an uneven distribution of chromosomes.
In a study published March 6 in the journal Cell, a team led by UW scientists reports on the workings of a key component of this machine. Named a kinetochore, it is a site on each chromosome that mechanically couples to spindle fibers.
"Kineochores are also regulatory hubs," the researchers noted. "They control chromosome movements through the lengthening and shortening of the attached microtubules. They sense and correct errors in attachment. They emit a "wait" signal until the microtubules properly attach." Careful control over microtubules, they added, is vital for accurate splitting of the chromosomes.
The lead researchers on the study were Andrew F. Powers and Andrew D. Frank from the UW Department of Physiology and Biophysics and Daniel R. Gestaut, from the UW Department of Biochemistry. The senior authors of the study were Charles "Chip" Asbury, assistant professor, and Linda Wordeman, associate professor, both of physiology and biophysics and both members of the UW Center for Cell Dynamics; and Trisha Davis, professor of biochemistry, and director of the Yeast Resource Center.
Asbury is known for research on molecular machines and motors, Wordeman for work on chromosome movement, and Davis for studies of spindle poles. All are part of the Seattle Mitosis Club led by Sue Biggins at the Fred Hutchinson Cancer Research Center.
To understand how the kinetochore functions, the scientists sought to uncover the basis for its most fundamental behavior: attaching microtubules. The most puzzling aspect of this attachment, according to the researchers, is that the kinetochore has to be strong yet dynamic. It has to keep a grip on the microtubule filaments even as they add and remove their subunits.
"This ability," the researchers said, "allows the kinetochore to harness microtubule shortening and lengthening to drive the movement of chromosomes."
The same coupling behavior is found in living things from yeast cells to humans, indicating that it was conserved during evolution as a good way of getting the job done.
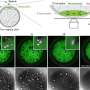
The question is how this mechanism works. Previous studies implicated a large, multiprotein complex, Ndc80, as a direct contact point between kinetochores and microtubules. However, researchers had only a static view of the complex. The UW researchers used special techniques to manipulate and track the activity of the complex in a laboratory set-up.
The researchers were able to show that the Ndc80 complex was indeed capable of forming dynamic, load-bearing attachments to the tips of the microtubules, probably by forming an array of individually weak microtubule binding elements that rapidly bind and unbind, but with a total energy large enough to hold on. The mechanism will produce a molecular friction that resists translocation of the microtubule through the attachment site. Other scientists have dubbed the mechanism a "slip clutch."
This kind of coupler, the researchers added, is able to remain continuously attached to the microtubule tip during both its assembly and disassembly phases. The coupler also can harness the energy released during disassembly to produce mechanical force. Coupling may depend on positively charged areas on the complex that interact with negatively charged hooks on the microtubules by electrostatic force.
Based on their findings, the scientists propose arrays of Ndc80 complexes supply the combination of plasticity and strength that allows kinetechores to hold on loosely but not let go of the tips of the microtubules.
Source: University of Washington