Researchers See Complex Atomic Choreography as Crystals Melt
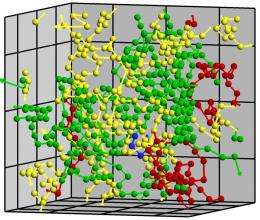
(PhysOrg.com) -- Conga lines of atoms wend their way through a crystal, their numbers growing as more and more atoms join the migration. The worm-like lines of atoms randomly converge, forming tangles that evolve into droplets of liquid that signal the beginning of the complicated process known as melting.
That’s the picture painted by Georgia Tech researchers, who used molecular dynamics simulations to study how melting takes place deep within a perfect crystal. Reported in the April 2008 issue of the journal Physical Review B, the research offers a new and highly detailed look at a complex phenomenon that has intrigued theoreticians for nearly a century.
“Atoms start to vibrate, and then they find a buddy with the same vibration, attitude and direction,” explained Mo Li, a Georgia Tech School of Materials Science and Engineering professor who led the study. “They then start to move together and form this line that resembles a worm. When you generate a lot of worms in a crystalline system over time, they start to tangle. That’s when homogenous melting starts.”
Solid materials ordinarily melt when the combination of entropy and energy favors the liquid phase. Melting most often starts on the surface, or where crystalline defects and boundaries create weak bonds that allow vibrations to shake atoms loose from the structure.
But with the advent of material-cutting lasers that can heat crystalline materials from the inside - and with growing interest in high-pressure geothermal melting that also takes place from the inside - scientists needed to understand how non-surface melting happens.
It stands to reason that melting within a crystal would be very different from surface melting, Li points out. Atoms constrained within a perfect crystal have no place to go unless an adjacent atom moves to create a temporary opening in the crystalline structure. But if that happens, perhaps when one atom temporarily jumps into this vacant space, whole lines of atoms can begin to move in concert, each occupying the space vacated by its neighbor.
But observing that movement in detail hasn’t been easy.
Simulating the action of millions of atoms requires considerable computing time, so to minimize processor load, earlier simulations had raised the temperature of crystals rapidly, causing them to melt catastrophically. Li and his collaborator, former graduate student Xian-Ming Bai - now a postdoctoral fellow at Northwestern University - decided to slow the process down to observe what would happen with more realistic scenarios in which the temperature rises gradually over time.
“If you heat the atoms too quickly, they have no opportunity to explore low-energy configurations,” Li explained. “We wanted to slow down and look at how the thermodynamics works. We heated the material to a specific temperature and then held it for as long as we could to let the system evolve - like marinating meat for better flavor.”
Using a large computer cluster in Georgia Tech’s School of Materials Science and Engineering, the researchers studied what would happen to a crystal composed of several thousand argon atoms as it was heated. Argon was chosen because its physical behavior has been thoroughly studied. To form a continuous crystal without surfaces, the researchers mathematically joined the opposite faces of the simulated crystal.
Their patience was rewarded by a new and different view of the choreography behind this common phase transition. Beyond the basic scientific interest, those revelations could lead to a better understanding of materials processing.
“The results point toward some very interesting applications in controlling and manipulating melting,” says Li. “Since atoms don’t move individually, perhaps we can do something to change the behavior of this melting.”
At this point, Li doesn’t know if the dancing atoms observed in the simulated argon crystal are typical of melting in atomic crystals. He would like to study other systems, and expand the work to include more complex structures such as ceramics, metallic alloys, polymers and semiconducting materials such as silicon.
“Regardless of the structure, I believe the general principle of melting will be the coordinated motion of atoms or molecules,” he added. “Atoms and molecules are bound together in certain directions and they would have to move in certain directions. The consequence is that they will have to dance together.”
Ironically, the research into melting began with a study of how materials freeze, a project sponsored by the Defense Advanced Research Projects Agency (DARPA). Toward the end of that project, Li and his collaborators decided to reverse the process to see what would happen to the crystals they had created.
“This gives us a reference point for understanding what is really happening in nature,” he says. “There are still a lot of open questions, but no one really imagined that a mechanism like this existed.”
Provided by Georgia Institute of Technology