Researchers Unlock Molecular Origin of Blood Stem Cells
(PhysOrg.com) -- A research team led by Nancy Speck, PhD, Professor of Cell and Developmental Biology at the University of Pennsylvania School of Medicine, has identified the location and developmental timeline in which a majority of bone marrow stem cells form in the mouse embryo. The findings, appearing online this week in the journal Nature, highlight critical steps in the origin of hematopoietic (or blood) stem cells (HSCs), says senior author Speck, who is also an Investigator with the Abramson Family Cancer Research Institute at Penn.
Because HSCs, found in the bone marrow of adult mammals, generate all of the blood cell types of the body, unlocking the secrets of their origin may help researchers to better manipulate embryonic stem cells to generate new blood cells for therapy.
“The ultimate goal for stem cell therapies is to take embryonic stem cells and push them down a particular lineage to replace diseased or dead cells in human adults or children,” says Speck. For instance, in theory embryonic stem cells could be tweaked in a lab to provide a patient with bone marrow failure a fresh supply of compatible HSCs.
To date, however, Speck says scientists have been unable to coax embryonic stem cells to become HSCs without significant genetic manipulations that are too risky for clinical therapies. First things first, Speck says: “You have to understand what's happening in the embryo.”
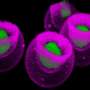
Previous studies hinted that HSCs originated from a small population of cells lining the blood vessels, called endothelial cells. But, it was unclear how endothelial cells transitioned to blood stem cells during early development.
Before joining Penn in September 2008, Speck, then at Dartmouth Medical School, led a team that confirmed that HSCs in bone marrow were originating from the endothelial cells and determined whether the activity of a protein called Runx1, which is known to be critical in the formation of blood cells, was responsible for this important transition.
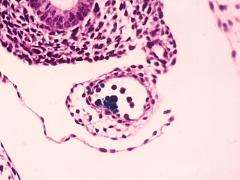
First, the researchers inactivated the gene that codes for the protein Runx1 in the endothelial cells of mouse embryos. During development, some endothelial cells express Runx1, signaling the production of grapelike clusters of HSCs along the interior walls of several major blood vessels. Upon release from the vessel walls HSCs enter the blood circulation and travel to the fetal liver, and upon birth they relocate to the bone marrow.
By selectively blocking the ability of endothelial cells to express Runx1 during embryo development, the researchers halted HSC production, demonstrating that Runx1 is vital to the endothelial cell to HSC transition.
Next, Speck’s team shut off Runx1 expression in mouse embryos at day 11.5 of gestation -- a time when most newly born HSCs have detached from the vessel wall and migrated to the fetal liver. The researchers found that blocking Runx1 expression had no effect on HSC formation, suggesting while Runx1 is required for the transition from endothelium to HSCs, the process is complete by the end of the 11th day of gestation.
The researchers also showed that at least 95 percent of all adult HSCs (and therefore almost all adult blood) originate in the endothelium, during this short window of time during development.
“This study helps illustrate a very important step in the transitional stage from embryonic stem cells to HSCs - the need to move through endothelial cells as an intermediary,” Speck says.
Understanding the location and developmental timeline of the origin of blood stem cells will help guide future efforts to coax embryonic stem cells to produce mature blood cells, she says.
Co-authors include Michael Chen and Brandon Zeigler from Dartmouth Medical School (Departments of Biochemistry and Genetics) and Tomomasa Yokomizo and Elaine Dzierzak from Erasmus Medical Center in Rotterdam, Netherlands.
Provided by University of Pennsylvania